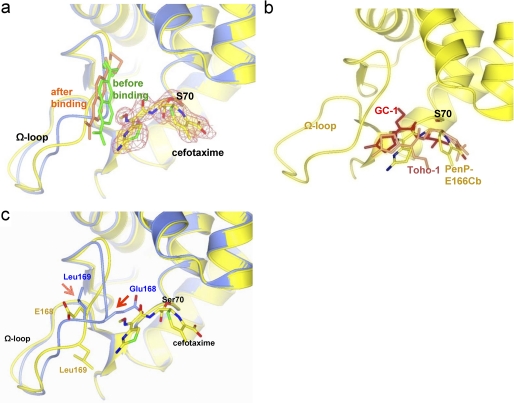
FIGURE 4.
The structure of PenP-E166Cb with cefotaxime acylated at its active site. a, cefotaxime is acylated to Ser-70 at the active site of PenP-E166Cb. The PenP-E166Cb-cefotaxime complex structure (in yellow with its BADAN molecule in gold) is aligned with that of PenP-E166Cb (blue with its BADAN molecule in green). The cefotaxime molecule is depicted in cpk color scheme. The fo-fc map of cefotaxime is contoured at 2.0 σ. b, comparison of the binding mode of cefotaxime in PenP-E166Cb (cpk color) with that in Toho-1 (coral) and GC1 (red). The protein structure of PenP-E166Cb is in yellow. c, induced conformational change of the Ω-loop upon acylation of cefotaxime. The PenP-E166Cb-cefotaxime complex structure (yellow) is aligned with that of PenP-E166Cb (blue). Residues on the Ω-loop (E166, E166Cb, and P167) and key catalytic residues for acylation (K73, S70, and S130) are displayed in cpk color scheme. The BADAN fluorophore is omitted for clarity purpose. The arrows indicate the induced conformational change of certain residues.