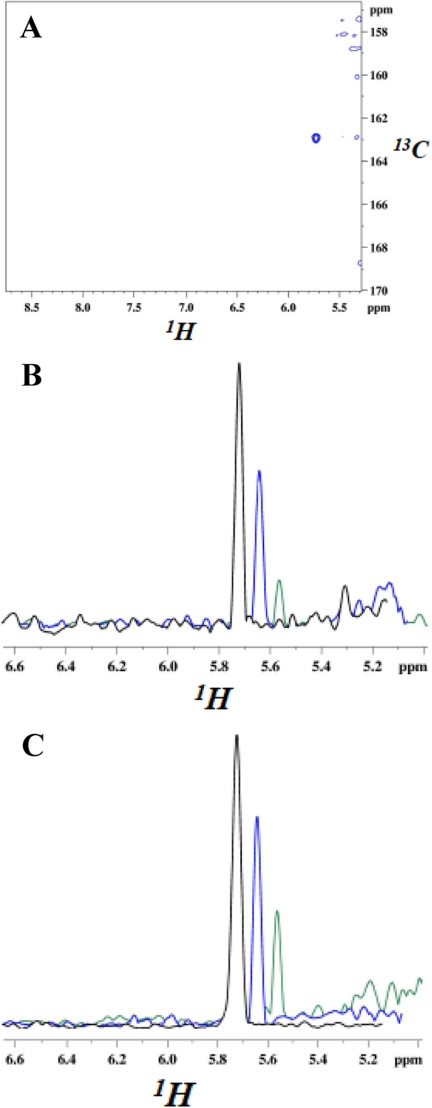
FIGURE 3.
NMR resonances from carbamate moiety of N-carboxylated Lys-392 and their decrease upon the addition of two different penicillins. Spectra were recorded at 295 K, 16.4 T. A, cross-peak from the NζH bond of the carbamate moiety. U-15N/80%2H-labeled BlaRS in buffer containing 13C bicarbonate was used as the CO2 source. The two-dimensional spectrum is a 1H-13CO projection of the three-dimensional HNCO, and the single cross-peak (blue) has the carbamate 13CO and 1H chemical shifts. The faint contours on the right are residual water signals. B and C, cross-sections of 1H-15N heteronuclear single quantum correlation spectra showing the decrease of the carbamate 1H resonance after the addition of antibiotic. B, penicillin G addition at 0 min (black, 100%), + 2 h (blue, 60%), and + 36 h (green, 20%). C, CBAP addition at 0 min (black, 100%), + 2 h (blue, 72%), and + 36 h (green, 30%). The decarboxylation is slower than that observed in previous kinetic measurements (9), reflecting the use of the higher protein concentration (150 μm) for NMR versus kinetic (1 μm) experiments.