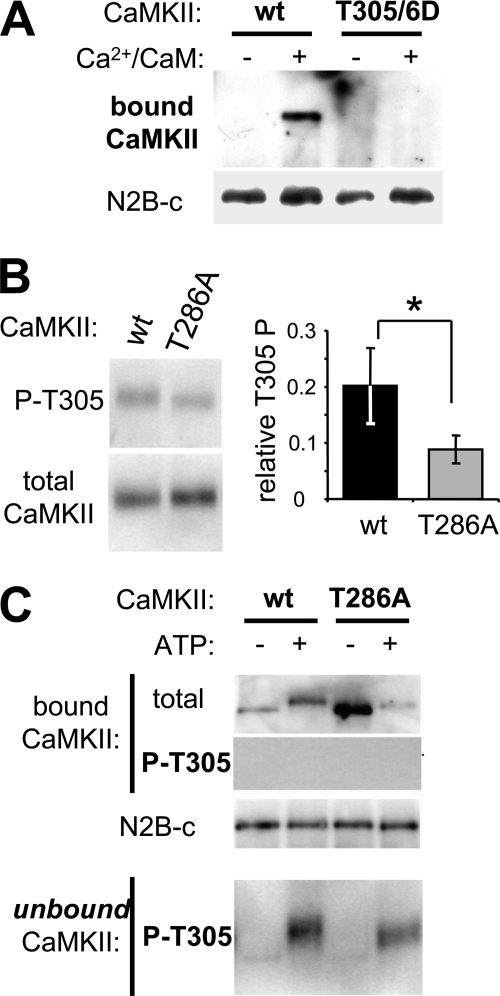
FIGURE 5.
T305/6 autophosphorylation inhibits CaMKII binding to GluN2B. A, Ca2+/CaM stimulated in vitro GluN2B binding of GFP-CaMKII wild type but not of its T305/6D phospho-mimetic mutant, as determined by Western blot analysis of the protein complex. B, Ca2+/CaM stimulation induced more T305 autophosphorylation in GFP-CaMKII wild type compared with its T286A mutant (*, p < 0.05, t test), as determined by quantification (right; n = 3) of Western blot analysis with phospho-T305 selective antibody (left). C, in a GFP-CaMKII binding reaction to immobilized N2B, CaMKII autophosphorylation at T305 was detected only in the unbound fraction, not in the N2B-bound CaMKII, as detected by Western blot analysis of the protein complex and the unbound supernatant.