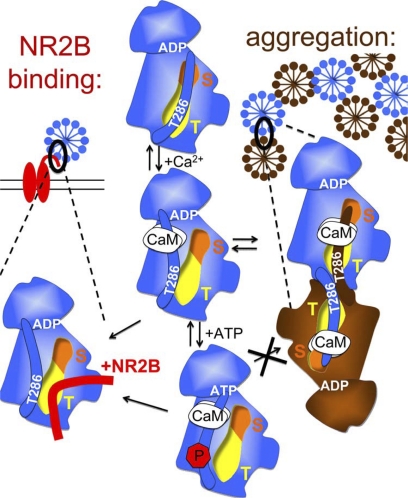
FIGURE 9.
Schematic model: CaMKII binding to GluN2B (left) and aggregation of multiple CaMKII holoenzymes (right) are based on similar molecular interaction mechanisms. Displacement of the auto-inhibitory α-helix (which includes the region around T286) by Ca2+/CaM binding activates the kinase (by allowing substrate access to the S-site; orange) and exposes the T-site (yellow), which then can interact with other binding partners: either the region around S1303 in GluN2B (red) or the T286 region of a kinase subunit on another holoenzyme (brown). Both interactions are enhanced by nucleotide binding to CaMKII. However, while T286 autophosphorylation further enhances binding to GluN2B, it inhibits aggregation of holoenzymes.