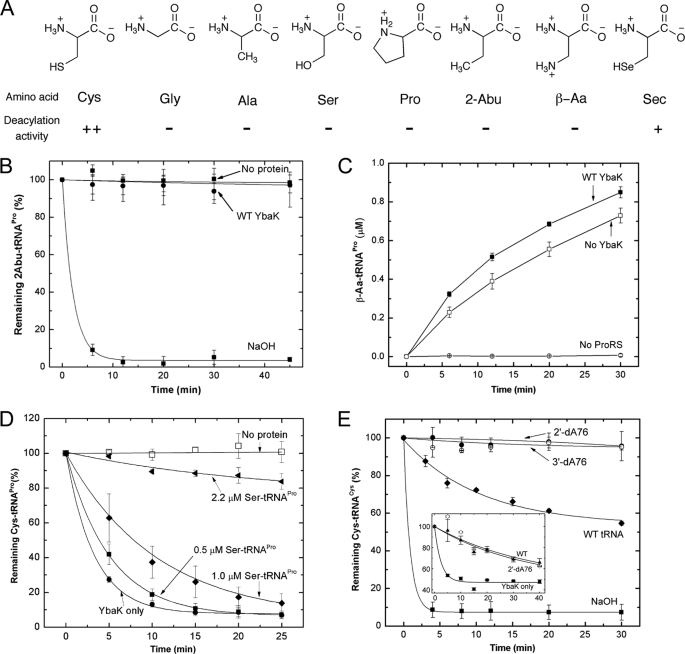
FIGURE 4.
Substrate specificity of H. influenzae YbaK. A, structures of amino acids tested as substrates for post-transfer editing by H. influenzae YbaK. ++, hydrolyzed; +, hydrolyzed (deacylation activity is ∼10-fold reduced relative to Cys-tRNA); −, not hydrolyzed (deacylation activity >100-fold reduced relative to Cys-tRNA). B, deacylation of 2-Abu-tRNAPro by H. influenzae YbaK (0.5 μm). Hydrolysis in the absence of protein or in the presence of 2 m NaOH is also shown. C, mischarging of β-Aa onto 10 μm tRNAPro by 1.5 μm E. coli K279A ProRS in the absence or presence of WT H. influenzae YbaK (2 μm). D, YbaK (0.1 μm) deacylation of 0.5 μm Cys-tRNAPro in the absence (YbaK only) and presence of increasing concentrations of Ser-tRNAPro. E, deacylation of 2′-dA76 or 3′-dA76 Cys-tRNACys by WT H. influenzae YbaK (1 μm). Hydrolysis of WT Cys-tRNACys by YbaK or 2 m NaOH is also shown. Inset, deacylation of [35S]Cys-tRNACys by E. coli YbaK (0.5 μm) in the absence (YbaK only) and presence of Cys-tRNACys (WT) and Cys-2′-dA76-tRNACys (2′dA76) at 30 °C. Axis labels are the same as in the main panel. Error bars, S.D.