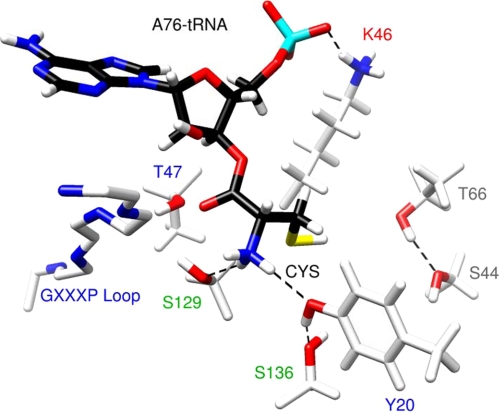
FIGURE 8.
Summary of interactions in the substrate binding pocket of H. influenzae YbaK based on computational and experimental results. A polar environment formed by residues Thr-66, Ser-44, and Ser-136 may lower the pKa of the cysteine thiol. The thiolate thus generated attacks the carbonyl center, leading to the cleavage of the ester bond and formation of a four-membered cyclic thiolactone intermediate. The tetrahedral oxyanionic transition state is stabilized by Thr-47, Ser-129, and the 2′-OH of A76 of the tRNA. Tyr-20 is involved in a hydrogen bonding network with the substrate amine group and the hydroxyl group of Ser-136. The universally conserved Lys-46 residue stabilizes the proper substrate orientation via interaction with the backbone A76 phosphate. The most critical residues or functional groups are labeled in red type. Alanine substitution resulted in deacylation efficiency decreases of ≥66-fold (red), 21–45-fold (blue), 4–6-fold (green), and ≤2-fold (gray).