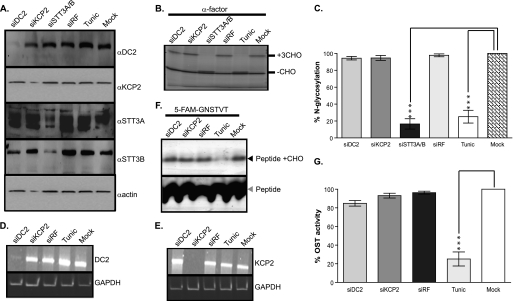
FIGURE 2.
DC2 and KCP2 are dispensable for N-glycosylation activity of the OST complex. A, HeLa cells treated with siRNA duplexes for DC2 (siDC2), KCP2 (siKCP2), STT3A and STT3B (siSTT3A/B), or a siRisc-free control (siRF) or mock-transfected (Mock). HeLa cells were also incubated overnight with 20 μg/ml tunicamycin (Tunic). Western blots were performed after treatments with antibodies against DC2, KCP2, STT3A, STT3B, and β-actin. B, S. cerevisiae α-factor was synthesized as a radiolabeled polypeptide using a rabbit reticulocyte lysate system supplemented with semipermeabilized HeLa cells prepared 72 h after transfection with siRNAs specific for the mRNAs encoding DC2 (lane 1), KCP2 (lane 2), or STT3A/B (lane 3), or a non-functional control siRNA (siRF) (lane 4) or following mock transfection (lane 6). As a positive control for loss of N-glycosylation, HeLa cells were incubated with 20 μg/ml tunicamycin overnight prior to isolation on day 2 (lane 5). The resulting glycosylated (+CHO) and non-glycosylated (−CHO) polypeptides are shown. C, the relative proportion of glycosylated polypeptide was calculated for each sample and expressed as a percentage of the total protein recovered. The values expressed in the graph are the mean ± S.E. (error bars) of three independent experiments. Levels of N-glycosylation that differ from the mock-treated control with a significance of p < 0.001 (***) are indicated by asterisks. D and E, HeLa cells treated with siRNA duplexes for DC2 (siDC2) and KCP2 (siKCP2) or a siRisc-free control (siRF) or mock-transfected (Mock). HeLa cells were also incubated overnight with 20 μg/ml tunicamycin (Tunic). RT-PCR was performed on RNA isolated after the above treatments with primers specific for DC2 and GAPDH (D) or KCP2 and GAPDH (E). F, 5-carboxyfluorescein-GNSTVT peptide was added to the reaction mix containing free lipid-linked oligosaccharide donor, LLO buffer supplemented with semipermeabilized HeLa cells prepared 72 h after transfection with siRNAs specific for the mRNAs encoding DC2 (lane 1) or for KCP2 (lane 2) or a non-functional control siRNA (siRF) (lane 3) or following mock transfection (lane 5). As a positive control for loss of N-glycosylation, HeLa cells were incubated with 20 μg/ml tunicamycin overnight prior to isolation on day 3 (lane 4). The reaction was separated through a 15–25% gradient gel and analyzed using LAS3000 using a blue LED. The non-modified peptide (Peptide) and resulting glycosylated peptide products (Peptide +CHO) are shown. G, the resulting peptide products and the proportion of N-glycosylated products obtained after various treatments are indicated as described above.