Abstract
The Rab27 effector granuphilin/Slp4 is essential for the stable attachment (docking) of secretory granules to the plasma membrane, and it also inhibits subsequent fusion. Granuphilin is thought to mediate these processes through interactions with Rab27 on the granule membrane and with syntaxin-1a on the plasma membrane and its binding partner Munc18-1. Consistent with this hypothesis, both syntaxin-1a- and Munc18-1-deficient secretory cells, as well as granuphilin null cells, have been observed to have a deficit of docked granules. However, to date there has been no direct comparative analysis of the docking defects in those mutant cells. In this study, we morphometrically compared granule-docking states between granuphilin null and syntaxin-1a null pancreatic β cells derived from mice having the same genetic background. We found that loss of syntaxin-1a does not cause a significant granule-docking defect, in contrast to granuphilin deficiency. Furthermore, we newly generated granuphilin/syntaxin-1a double knock-out mice, characterized their phenotypes, and found that the double mutant mice represent a phenocopy of granuphilin null mice and do not represent phenotypes of syntaxin-1a null mice, including their granule-docking behavior. Because granuphilin binds to syntaxin-2 and syntaxin-3 as well as syntaxin-1a, it likely mediates granule docking through interactions with those multiple syntaxins on the plasma membrane.
Keywords: Docking, Electron Microscopy (EM), Exocytosis, Insulin Secretion, Intracellular Trafficking, Membrane Trafficking, Pancreatic Islets, Rab Proteins, Secretion, Regulated Exocytosis
Introduction
In the constitutive secretory pathway that operates in all cells, transport vesicles leave the trans Golgi network in a steady stream, and the amount of the protein released largely depends on their synthesis rate. By contrast, specialized secretory cells have another secretory pathway, in which soluble proteins are initially stored in secretory vesicles and released only in the presence of an appropriate secretagogue. In this regulated secretory pathway, the rate-limiting step lies in one of the exocytic steps from the intracellular transport to the final fusion of the secretory vesicle membrane to the plasma membrane, which allows a rapid secretory response to extracellular stimulation. Although secretory vesicles must eventually be transported close to the plasma membrane for release, some vesicles are already attached to the plasma membrane. Those stably docked vesicles are thought to constitute a readily releasable pool, because vesicles such as synaptic vesicles must fuse within 1 ms after stimulation. However, exocytosis of secretory granules occurs much slower, at about 1–10 s (1), and the granules that fused first may not necessarily be derived from the docked granules. In fact, living cells have been observed to permit fusion without prior pausing at the plasma membrane in an early secretory phase (2, 3).
It is important to elucidate the molecular mechanism for the stable docking of secretory vesicles to the plasma membrane because this phenomenon is a unique hallmark of regulated exocytosis. In all intracellular pathways other than regulated exocytosis, the attachment of the vesicular membrane to the targeting membrane instantly leads to constitutive fusion, and therefore, vesicles stably docked to the target membrane can barely be discerned in static electron micrographs. Although docked vesicles in regulated exocytosis appear to be poised for release upon secretagogue sensing, they may result from the inhibitory nature of this secretory pathway, such that incoming vesicles would not fuse spontaneously (4, 5). We previously demonstrated that the Rab27 effector, granuphilin/Slp4, is essential for the stable docking of secretory granules in pancreatic β cells (6) and pituitary endocrine cells (7). Granuphilin-deficient cells show a specific loss of the granules directly attached to the plasma membrane under the electron microscope. Nevertheless, they exhibit elevated secretion in both the basal and stimulated states (3, 6). Granuphilin specifically interacts with a closed form of syntaxin-1a, a soluble N-ethylmaleimide-sensitive factor attachment protein receptors, on the plasma membrane (8). This form of syntaxin-1a can interact with Munc18-1 but not with other soluble N-ethylmaleimide-sensitive factor attachment protein receptors to execute a fusion reaction (9). Thus, granuphilin stabilizes the fusion-incompetent syntaxin-1a·Munc18-1 complex and inhibits subsequent fusion of docked granules. The absence of granuphilin and of stably docked granules may allow undocked granules to give easier access to fusion-competent syntaxin-1a or to free plasma membrane, which could increase the efficiency of fusion from undocked granules. Consistent with the model that granuphilin mediates the docking process by linking Rab27a/b on the granule membrane (10, 11) and the syntaxin-1a· Munc18-1 complex on the plasma membrane (8, 12), loss of Munc18-1 leads to a docking defect in the secretory granules in chromaffin cells (13). Furthermore, syntaxin-1a null β cells have recently been reported to exhibit a granule-docking defect (14). However, direct quantitative comparison of granule-docking defects among those mutant cells has never been performed.
In this study, we morphometrically compared intracellular insulin granule distributions between granuphilin null and syntaxin-1a null β cells derived from mice of identical genetic background. Furthermore, to genetically determine the functional relationship between the two molecules, we newly generated granuphilin/syntaxin-1a double mutant mice and compared their phenotypes with those of each single mutant mouse. We found that syntaxin-1a null cells do not show a significant docking defect, in contrast to granuphilin null cells. Furthermore, the double mutant cells have a docking defect comparable with that of granuphilin null cells. Protein interaction analyses indicate that granuphilin binds to syntaxin-2 and syntaxin-3 as well as syntaxin-1a but not to syntaxin-4. These findings suggest redundant and differential roles of plasma membrane syntaxin isoforms in granule docking and exocytosis.
EXPERIMENTAL PROCEDURES
Animal Procedures
All animal experiments were performed in accordance with the rules and regulations of the Animal Care and Experimentation Committee, Gunma University. All mice had ad libitum access to water and standard laboratory chow (CE-2; CLEA Japan, Tokyo, Japan) in an air-conditioned room with 12-h light-dark cycles. The granuphilin knock-out mice maintained in a C3H/He inbred background are described elsewhere (6). Syntaxin-1a knock-out mice with a C57BL/6 background (15) were backcrossed to a C3H/He background for 6–10 generations. Granuphilin/syntaxin-1a double knock-out mice were then obtained by mating the granuphilin knock-out mice with the syntaxin-1a knock-out mice.
Only male mice were phenotypically characterized in this study. An intraperitoneal glucose tolerance test (1 g/kg body weight) and an intraperitoneal insulin tolerance test (0.75 units of human insulin/kg of body weight) were performed as described elsewhere (16). Blood glucose levels were determined by a glucose oxidase method using a Glutest sensor and Glutest Pro GT-1660 (Sanwa Kagaku Kenkyujyo, Nagoya, Japan). The plasma insulin concentration was measured with an LBIS mouse insulin ELISA kit (U-type; Shibayagi, Shibukawa, Japan).
Antibodies, Immunoblotting, and Immunoprecipitation
The polyclonal anti-granuphilin antibody (αGrp-N) used here is described elsewhere (10). Mouse monoclonal antibodies toward Rab27a and Munc18-1 were purchased from BD Transduction Laboratories. Mouse monoclonal antibodies toward syntaxin-1 (HPC-1) and β-actin and rabbit polyclonal anti-FLAG antibody were purchased from Sigma. Rabbit polyclonal antibodies toward syntaxin-2 and syntaxin-3 were purchased from Synaptic Systems (Göttingen, Germany). Rabbit polyclonal anti-syntaxin-4 antibody was purchased from Millipore (Billerica, MA). Rat monoclonal anti-hemagglutinin (HA; 3F10) antibody was purchased from Roche Diagnostics.
Pancreatic islets were isolated from cervically dislocated mice by pancreatic duct injection of 500 units/ml collagenase solution (type XI; Sigma), followed by digestion at 37 °C for 25 min with mild shaking, and isolated islets were picked up by hand selection under a dissecting microscope, as described elsewhere (16). Protein extracts from islets were prepared and immunoblotted as described elsewhere (11). The immunoreactive bands were visualized using enhanced chemiluminescent Western blotting detection reagents (GE Healthcare). Chemiluminescent signals on the x-ray film were captured with an image scanner and quantified by ImageJ 1.31 software. For the immunoprecipitation analyses, cell extracts were prepared in 1 ml of lysis buffer (10 mm Tris, pH 7.5, 150 mm NaCl, 2 mm MgCl2, 0.5 mm EGTA, and 0.1% Triton X-100) and were centrifuged at 21,880 × g for 10 min. The supernatants were incubated with either 20 μl of rat anti-HA affinity matrix beads (Roche Diagnostics) or 30 μl of mouse anti-FLAG affinity gel (Sigma), with gentle agitation at 4 °C for 1 h.
Perifusion Assays in Isolated Islets
Isolated islets were cultured overnight in RPMI 1640 medium (11 mm glucose) supplemented with 5% fetal calf serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. Thirty islets were placed at the bottom of a 1-ml syringe that had been cut to a volume of 400 μl and plugged with cotton. They were perifused with standard low glucose Krebs-Ringer buffer (15 mm HEPES, pH 7.4, 120 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 24 mm NaHCO3, 0.1% bovine serum albumin, and 2.8 mm glucose) at a constant flow rate of 1.0 ml/min for 30 min. After this stabilization period, they were further perifused with the same buffer for 10 min followed by the buffer containing the secretagogues. All of the perifusate solution was equilibrated with 95% air and 5% CO2 and maintained at 37 °C. Fractions were collected every 1 min, and the insulin secretion was measured using an AlphaLISA insulin kit with an EnVision 2101 multilabel reader (PerkinElmer Life Sciences).
Electron Microscopic Analysis of Granule Distribution
Isolated islets were cultured overnight and then incubated in low glucose Krebs-Ringer buffer at 37 °C for 1 h. They were fixed by immersion with 2% paraformaldehyde, 2% glutaraldehyde, 0.2% picric acid in 0.1 m cacodylate buffer, pH 7.4, for 1.5 h at room temperature and embedded into 1% agarose. They were then postfixed, embedded in plastic resin, and sectioned. The ultrathin sections (80 nm) were analyzed under an electron microscope (JEM 1010; JEOL, Akishima, Japan) at an acceleration voltage of 80 kV. Micrographs were randomly taken at ×4000 magnification from 21 individual β cells from three mice for each genotype. The distance from the granule center to the plasma membrane was measured as described elsewhere (3).
Plasmid Construction
Full-length cDNAs encoding mouse Munc18-1–3 were amplified from mouse cDNAs by PCR using the following pairs of oligonucleotides with an EcoRI linker or an XhoI linker: 5′-GGGGAATTCATGGCCCCCATTGGCCTCA-3′ and 5′-GGGCTCGAGTTAACTGCTTATTTCTTCATCTG-3′ for Munc18-1; 5′-GGGGAATTCATGGCGCCCTTGGGGCTG-3′ and 5′-GGGCTCGAGTCAGGGCAGGGCCACACC-3′ for Munc18-2; and 5′-GGGGAATTCATGGCGCCGCCGGTATCG-3′ and 5′-GGGCTCGAGTTACTCATCCTTAAAGGAAACTT-3′ for Munc18-3. Purified PCR products were subcloned into the EcoRI and XhoI sites of pcDNA3.1/3×FLAG vector (17) and designated pcDNA3-FLAG-Munc18-1–3. Recombinant adenovirus bearing HA-tagged granuphilin cDNA was prepared as described elsewhere (10).
Cell Culture and Transfection
All cells were maintained in a humidified incubator with 5% CO2 at 37 °C. Mouse insulinoma MIN6 cells and HEK293A cells were cultured in high glucose (25 mm) Dulbecco's modified Eagle's medium supplemented with 15% fetal calf serum and 55 μm 2-mercaptoethanol and with 10% fetal calf serum, respectively. The pcDNA3-FLAG-Munc18-1–3 and pcDNA3-HA-granuphilin-a vectors (a total of 8 μg of plasmids) were transfected into HEK293A cells using Lipofectamine 2000 reagent (Invitrogen). Two days after transfection, cells were harvested and homogenized for immunoprecipitation.
RESULTS
Generation of Granuphilin and Syntaxin-1a Double Knock-out Mice
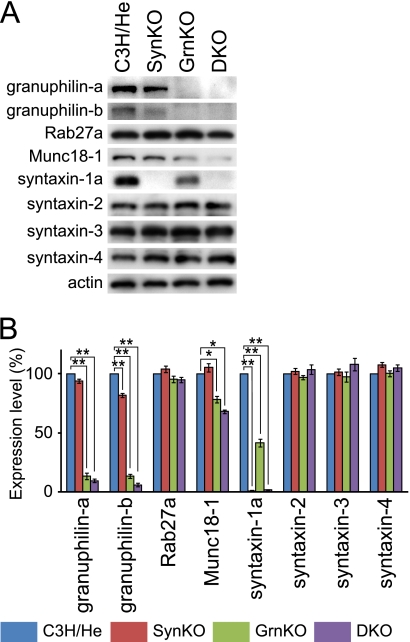
To genetically investigate the functional relationship between granuphilin and syntaxin-1a, we generated double mutant mice by crossing granuphilin knock-out mice with syntaxin-1a knock-out mice. All the single and double mutant mice had a C3H/He genetic background. We first isolated pancreatic islets and examined the expression levels of those two proteins and their related molecules (Fig. 1). The syntaxin-1a knock-out mice showed decreased levels of granuphilin-b (82%) but a normal level of Munc18-1, compared with the control C3H/He mice. However, the granuphilin knock-out mice showed reduced levels of syntaxin-1a (42%) and Munc18-1 (78%), as reported elsewhere (6). Similarly, double knock-out mice exhibited a reduced level of Munc18-1 (68%). We also investigated the expression levels of other syntaxins that reside on the plasma membrane (18). Because syntaxin-1b is not present in pancreatic β cells or in syntaxin-1a null β cells (14), we examined syntaxin-2–4. However, in contrast to syntaxin-1a, none of them showed changes in expression levels in the single or double mutant islets. These findings support the previous findings that granuphilin preferentially interacts with syntaxin-1a and Munc18-1 (8, 12).
FIGURE 1.
Protein expression in pancreatic islets. A, protein extracts (5 μg) from pancreatic islets of control C3H/He, syntaxin-1a knock-out (SynKO), granuphilin knock-out (GrnKO), and granuphilin/syntaxin-1a double knock-out (DKO) mice were electrophoresed for immunoblotting with antibodies toward the indicated proteins. B, protein expression levels were quantified from three independent experimental preparations. The band intensity of each protein was normalized by that of β-actin. All results are provided as the mean ± S.E. *, p < 0.05; **, p < 0.001 versus C3H/He.
In Vivo Phenotypes of Double Mutant Mice
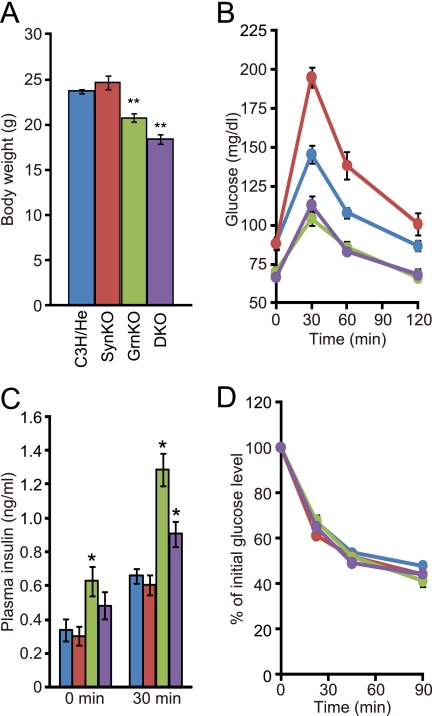
As was the case with the single mutant mice (6, 15), the double mutant mice were both viable and fertile and showed no gross abnormalities in development or behavior. As shown in Fig. 2A, syntaxin-1a null mice had normal body weight, whereas granuphilin null mice had reduced weight, as reported elsewhere (6). The double mutant mice also showed a decrease comparable with that of the granuphilin null mice. Consistent with the previous findings (6, 14), granuphilin null mice had lower blood glucose levels both in fasting mice and after a glucose load, whereas syntaxin-1a null mice showed higher glucose levels after a glucose load, compared with control mice (Fig. 2B). Despite the elevated glucose levels in syntaxin-1a mutant mice, the double mutant mice exhibited lower blood glucose levels, being nearly identical to those of the granuphilin null mice. Plasma insulin concentrations were higher in the granuphilin null and double mutant mice (Fig. 2C). Insulin tolerance tests revealed that all the mutant mice had normal insulin sensitivity (Fig. 2D). These findings indicate that double mutant mice represented a phenocopy of granuphilin single mutant mice.
FIGURE 2.
In vivo phenotypes of granuphilin and/or syntaxin-1a knock-out mice. A, body weight. B, blood glucose concentrations during an intraperitoneal glucose tolerance test. C, plasma insulin concentrations before and 30 min after a glucose load. D, percentage of starting blood glucose concentration during an intraperitoneal insulin tolerance test. Each measurement was performed in age-matched (9–16-week-old), C3H/He (blue; n = 14 for A, B, and D and n = 8 for C), SynKO (red; n = 11 for A, B, and D and n = 9 for C), GrnKO (green; n = 10 for A, B, and D and n = 9 for C), and DKO (purple; n = 11 for A, B, and D and n = 9 for C) mice. All results are provided as the mean ± S.E. The statistical significance of differences between means was assessed by a repeated measure of analysis of variance (Tukey-Kramer's method). *, p < 0.05; **, p < 0.001 versus C3H/He.
Insulin Secretion from Perifused Islets
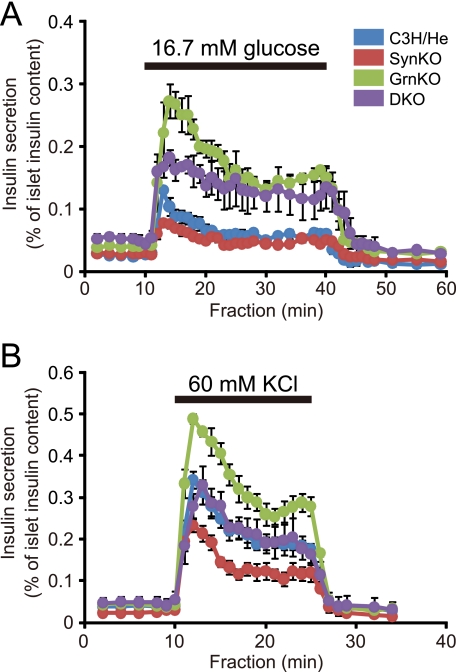
To directly examine the insulin secretion ability, we performed perifusion analyses in isolated islets. Consistent with the previous finding (14), syntaxin-1a null islets showed decreased insulin secretion in the first 7 min of 16.7 mm glucose stimulation (Fig. 3A; p < 0.05 versus control islets). By contrast, the granuphilin null islets exhibited enhanced insulin secretion throughout the 30 min of glucose stimulation (p < 0.01), as shown previously (6). The double mutant islets also showed enhanced insulin secretion in both the early and late phases (p < 0.05), although they tended to show statistically insignificant, lower insulin secretion especially in the early phase compared with the granuphilin single mutant islets.
FIGURE 3.
Insulin secretion profiles in perifused islets. Islets isolated from age-matched (20–30-week-old), C3H/He (blue; n = 4 for A and n = 9 for B), SynKO (red; n = 3 for A and n = 8 for B), GrnKO (green; n = 4 for A and n = 8 for B), and DKO (purple; n = 3 for A and n = 8 for B) mice were perifused with standard 2.8 mm glucose-containing Krebs-Ringer buffer for 30 min. Thereafter, the collection of each fraction (1 ml/min) was started, and an appropriate secretagogue was applied at 10 min after the collection. For glucose stimulation (A), islets were perifused with the buffer containing 16.7 mm glucose for 30 min followed by the standard 2.8 mm glucose buffer for 20 min. For stimulation by high K+ concentration (B), islets were perifused with buffer containing 60 mm KCl plus 65 mm NaCl for 15 min followed by the standard buffer for 10 min.
Because syntaxin-1a deficiency selectively affected the early phase of insulin secretion, we then exposed the islets to high K+ stimulation, which artificially induces depolarization-induced Ca2+ mobilization as in the early phase of glucose stimulation. The stronger stimulation by 60 mm KCl revealed a more prominent decrease in secretion in the syntaxin-1a null islets (p < 0.05 versus control islets; Fig. 3B). By contrast, the granuphilin null islets again showed increased secretion (p < 0.05), as reported elsewhere (6). In this case, however, the double mutant islets reflected the defect due to syntaxin-1a deficiency and exhibited an intermediate secretion level between the two single mutant islets, which was comparable with that of wild-type cells. Thus, although double mutant β cells exhibit a glucose-induced insulin secretory response similar to that of granuphilin null cells, they show a lower response to strong depolarization stimulation.
Morphometric Analyses of Insulin Granule Distributions
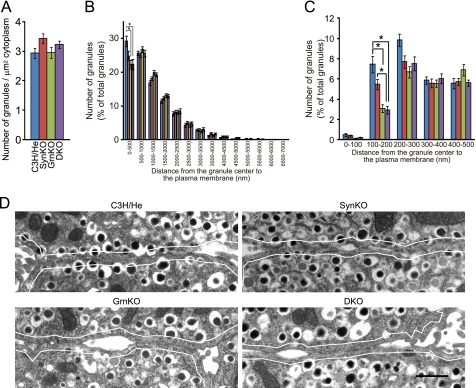
Electron microscopy has demonstrated that granuphilin null β cells exhibit a specific deficit of granules having centers within 200 nm of the plasma membrane (6). Because the diameter of insulin granules is ∼350 nm, this indicates that granuphilin is essential for the direct attachment (docking) of an insulin granule to the plasma membrane. It has also been reported that syntaxin-1a null β cells show a drastically reduced number of granules at a distance of <10 nm from the plasma membrane (14). Because granuphilin interacts with syntaxin-1a (8), both molecules may mediate granule docking in the same pathway. If so, those single and double mutant β cells should show quantitatively similar docking defects. To test this hypothesis, all those mutant islets as well as the wild-type islets were processed for and examined by electron microscopy under the same conditions. The electron micrographs were then morphometrically analyzed as described elsewhere (3, 6). We counted all the granules in the cytoplasm and found that the average granule density was unchanged among wild-type and the three kinds of mutant cells (Fig. 4A). When those granules were categorized according to their distance from the granule center to the plasma membrane, at 500-nm increments, there were no differences in the number of granules, except for that of granules within 500 nm of the plasma membrane (Fig. 4B). The granuphilin null and double mutant cells, but not the syntaxin-1a null cells, showed a significantly reduced number of granules within 500 nm (p < 0.05 versus C3H/He cells). When those granules within 500 nm were further categorized at 100-nm intervals, the number of granules at 100–200 nm was specifically decreased in granuphilin null and double mutant cells (Fig. 4C; p < 0.05 versus C3H/He cells). By contrast, syntaxin-1a null cells showed no significant change of granule distributions, although they tended to have a decreased number of granules at 100–200 nm. Importantly, double mutant cells showed a decrease in granules at 100–200 nm even compared with syntaxin-1a null cells (p < 0.05). As can be seen in the actual electron micrographs (Fig. 4D), syntaxin-1a null β cells contained a significant number of granules directly attached to the plasma membrane. By contrast, these sort of docked granules were missing in both the granuphilin null and double mutant cells. These findings clearly demonstrate that syntaxin-1a deficiency does not cause a distinguishable granule-docking defect of the sort seen in granuphilin deficiency, contrary to a previous report (14).
FIGURE 4.
Electron microscopic analysis of the distribution of insulin granules. Islets isolated from 24- to 28-week-old, C3H/He (blue), SynKO (red), GrnKO (green), and DKO (purple) mice were incubated at 37 °C with 2.8 mm glucose-containing buffer for 1 h and then fixed. A, average granule density (granule number per cytoplasmic area). B and C, granules whose centers located within 7000 nm (B) and 500 nm (C) of the plasma membrane were categorized into 14 and 5 bins, respectively. All results are provided as the mean ± S.E. The statistical significance of differences between means was assessed by a repeated measure of analysis of variance (Tukey-Kramer's method). *, p < 0.05. D, electron micrographs of β cells from C3H/He, SynKO, GrnKO, and DKO mice. Solid lines indicate a 200-nm distance from the plasma membrane. Note that granules whose limiting membranes were directly attached to the plasma membrane are present in SynKO cells in contrast to GrnKO and DKO cells. Bar, 1 μm.
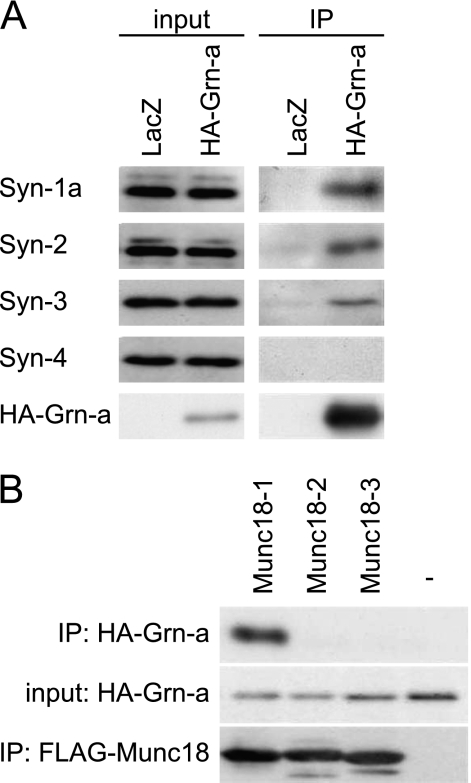
Granuphilin Interacts with Multiple Syntaxins on the Plasma Membrane
Because syntaxin-1a null β cells showed neither a clear docking defect nor a drastic secretory defect, we speculated that other syntaxin isoforms on the plasma membrane participate in the docking and fusion of insulin granules to the plasma membrane. In fact, syntaxin-4 has been shown to be involved in glucose-stimulated insulin secretion (19). We expressed HA-tagged granuphilin in the β cell line MIN6 and investigated whether it interacts with the endogenous syntaxins present on the plasma membrane. As found in pancreatic islets (Fig. 1), syntaxins 1a and -2–4 were all expressed in the MIN6 cells (Fig. 5A). Granuphilin interacted with syntaxin-2 and syntaxin-3, in addition to syntaxin-1a (8), but not with syntaxin-4, which is consistent with the previous finding in co-immunoprecipitation assay using COS-7 cells (20). Granuphilin is also known to interact with Munc18-1 (8, 12, 21). Because Munc18-1 specifically binds to syntaxin-1a, -2, and -3, but not to syntaxin-4 (22), the interaction of granuphilin with syntaxins may be mediated through its specific interaction with Munc18-1. To investigate this possibility, we simultaneously overexpressed granuphilin and Munc18 isoforms in HEK293A cells and examined the complex formation (Fig. 5B). Granuphilin selectively interacted with Munc18-1, but not with Munc18-2 or Munc18-3, the latter of which is known to specifically bind to syntaxin-4 (23). These findings suggest that granuphilin mediates granule docking through the interactions with complexes consisting of Munc18-1 and either syntaxin-1a, -2, or -3, and therefore, syntaxin-1a deficiency can be compensated for by using syntaxins 2 and 3, which does not cause a distinguishable granule-docking defect.
FIGURE 5.
Interaction between granuphilin-a with syntaxins 1–4 and Munc18-1–3 in MIN6 cells. A, MIN6 cells were infected with recombinant adenovirus encoding either LacZ or HA-tagged granuphilin-a (HA-Grn-a). The cell lysates were immunoprecipitated (IP) with anti-HA antibody, and the immune complexes along with 1:2000 volume of the original lysates (input) were immunoblotted with antibodies toward syntaxins 1–4 and HA. B, HEK293 cells were transfected with a plasmid encoding HA-Grn-a without (−) or with a plasmid encoding FLAG-Munc18-1–3. Proteins bound to anti-FLAG affinity gels were immunoblotted with antibodies toward HA and FLAG. Input corresponds to 1:200 volume of the original lysates.
DISCUSSION
We have shown that although syntaxin-1a null β cells tend to have a decreased number of insulin granules close to the plasma membrane, they show only a minor granule-docking defect, in contrast to a previous report (14). This discrepancy may reflect differences in the fixation protocol for the samples, the definition of docked granules, or in the genetic background of the mice. However, our simultaneous examination of mutant mice with the same genetic background clearly demonstrates that the docking defect in syntaxin-1a null β cells, if any, is much smaller than that in granuphilin null β cells. These findings underscore the importance of quantitatively assessing the granule-docking states under the same conditions among mutant cells. In fact, a recent study has also reported quantitative differences in docking defects between SNAP-25 null and Munc18-1 null chromaffin cells (24). Furthermore, some of the subtle docking phenotypes previously reported require advanced methodology and new docking definitions to become fully confirmed (25). Therefore, the morphological docking defects described in the literature may actually represent dysfunction during different molecular steps.
Lack of a clear docking defect in syntaxin-1a null cells indicates involvement of other syntaxins on the plasma membrane. The modest decrease of insulin secretion in syntaxin-1a null cells also suggests their involvement in the fusion of insulin granules. Thus, we investigated the possibility that granuphilin interacts with syntaxins residing on the plasma membrane and found that granuphilin interacts with syntaxin-2 and syntaxin-3 as well as syntaxin-1a, but not with syntaxin-4. Because the binding pattern of granuphilin to those syntaxins is identical to that of Munc18-1, granuphilin can interact with those syntaxins through Munc18-1, although direct interaction between granuphilin and syntaxin-1a is also reported (26). Whether granuphilin binds Munc18-1 and/or syntaxins, the syntaxins must be considered to be indispensable components for granule docking because Munc18-1 itself is not directly connected to the plasma membrane. It has been reported that granule docking is impaired in chromaffin cells expressing botulinum neurotoxin C and thus is syntaxin-dependent (27). However, it should be noted that this neurotoxin cleaves syntaxin-1–3 as well as SNAP-25 (28). Because a docking defect has also been reported in SNAP-25 null chromaffin cells by the same group (24), it has not been adequately determined whether any syntaxins are involved in granule docking. Besides SNAP-25, this finding simply indicates that all syntaxins, including syntaxin-1–3, can participate in the docking of chromaffin granules, as suggested for insulin granules based on these findings. Alternatively, the minor docking defect in the syntaxin-1a null β cells may indirectly result from the decreased expression level of granuphilin. It is also possible that granuphilin mediates granule docking through the phospholipid binding activity of its C2 domains, as found in another Rab27 effector, exophilin4, in pancreatic α cells (29). In those cases, no syntaxin would be involved in the docking process.
It is interesting that neither syntaxin-4 nor its partner, Munc18-3 (23), interacts with granuphilin, although pancreatic β cells of mice heterozygous for the syntaxin-4 mutation (19), or for the Munc18-3 mutation (30), do show significant decreases in insulin secretion. These findings suggest that those molecules are not involved in granule docking or possibly in the fusion of stably docked granules. Consistent with this hypothesis, Munc18-3 depletion selectively impairs the sustained phase of glucose-induced insulin release (31), in which most of the fusion is derived from undocked granules (2, 3, 32). By contrast, syntaxin-1a, 2, and 3 may be involved in the fusion of granuphilin-mediated docked granules. Consistently, granuphilin/syntaxin-1a double knock-out mice show enhanced glucose-induced insulin secretion despite the lack of syntaxin-1a, which suggests that syntaxin-1a is not actively involved in the fusion of undocked granules in the absence of granuphilin. However, it should be remembered that such differential roles of syntaxins in the exocytosis of docked and undocked granules do not necessarily account for their differential roles in the time course of secretion. In fact, the heterozygous mutation of syntaxin-4 has been reported to selectively affect insulin secretion in a relatively early period (within the first 7 min) after glucose stimulation (19). Furthermore, syntaxin-1a deficiency affects insulin secretion throughout a 15-min depolarization stimulation in the absence of docked granules in double mutant cells. Therefore, although docked and undocked granules may utilize different sets of fusion machinery, they do not fuse sequentially, but rather in parallel, after stimulation, as suggested and observed previously (3, 4), and any syntaxin can mediate the fusion of undocked granules in either an early or late phase of stimulus-induced secretion. Future studies should explore the differences between the molecular determinants of the rate-limiting exocytic steps for docked and undocked granules.
Acknowledgments
We thank T. Nara and T. Ushigome for their colony management of mice and J. Toshima for assistance in preparing the manuscript.
Footnotes
This work was supported by grants in aid for scientific research (to T. I. and R. I.), a Global COE program grant from Ministry of Education, Culture, Sports, Science and Technology of Japan, and in part by grants from the Mitsubishi Foundation and a Novo Nordisk insulin study award (to T. I.).
REFERENCES
- 1. Kasai H. (1999) Trends Neurosci. 22, 88–93 [DOI] [PubMed] [Google Scholar]
- 2. Shibasaki T., Takahashi H., Miki T., Sunaga Y., Matsumura K., Yamanaka M., Zhang C., Tamamoto A., Satoh T., Miyazaki J., Seino S. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 19333–19338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kasai K., Fujita T., Gomi H., Izumi T. (2008) Traffic 9, 1191–1203 [DOI] [PubMed] [Google Scholar]
- 4. Izumi T., Kasai K., Gomi H. (2007) Diabetes Obes. Metab. 9, Suppl. 2, 109–117 [DOI] [PubMed] [Google Scholar]
- 5. Izumi T. (2011) Front. Biosci. 16, 360–367 [DOI] [PubMed] [Google Scholar]
- 6. Gomi H., Mizutani S., Kasai K., Itohara S., Izumi T. (2005) J. Cell Biol. 171, 99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gomi H., Mori K., Itohara S., Izumi T. (2007) Mol. Biol. Cell 18, 4377–4386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Torii S., Zhao S., Yi Z., Takeuchi T., Izumi T. (2002) Mol. Cell. Biol. 22, 5518–5526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dulubova I., Sugita S., Hill S., Hosaka M., Fernandez I., Südhof T. C., Rizo J. (1999) EMBO J. 18, 4372–4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yi Z., Yokota H., Torii S., Aoki T., Hosaka M., Zhao S., Takata K., Takeuchi T., Izumi T. (2002) Mol. Cell. Biol. 22, 1858–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao S., Torii S., Yokota-Hashimoto H., Takeuchi T., Izumi T. (2002) Endocrinology 143, 1817–1824 [DOI] [PubMed] [Google Scholar]
- 12. Coppola T., Frantz C., Perret-Menoud V., Gattesco S., Hirling H., Regazzi R. (2002) Mol. Biol. Cell 13, 1906–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Voets T., Toonen R. F., Brian E. C., de Wit H., Moser T., Rettig J., Südhof T. C., Neher E., Verhage M. (2001) Neuron 31, 581–591 [DOI] [PubMed] [Google Scholar]
- 14. Ohara-Imaizumi M., Fujiwara T., Nakamichi Y., Okamura T., Akimoto Y., Kawai J., Matsushima S., Kawakami H., Watanabe T., Akagawa K., Nagamatsu S. (2007) J. Cell Biol. 177, 695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujiwara T., Mishima T., Kofuji T., Chiba T., Tanaka K., Yamamoto A., Akagawa K. (2006) J. Neurosci. 26, 5767–5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kasai K., Ohara-Imaizumi M., Takahashi N., Mizutani S., Zhao S., Kikuta T., Kasai H., Nagamatsu S., Gomi H., Izumi T. (2005) J. Clin. Invest. 115, 388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matsunaga K., Morita E., Saitoh T., Akira S., Ktistakis N. T., Izumi T., Noda T., Yoshimori T. (2010) J. Cell Biol. 190, 511–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Teng F. Y., Wang Y., Tang B. L. (2001) Genome Biol. 2, REVIEWS3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spurlin B. A., Thurmond D. C. (2006) Mol. Endocrinol. 20, 183–193 [DOI] [PubMed] [Google Scholar]
- 20. Fukuda M., Imai A., Nashida T., Shimomura H. (2005) J. Biol. Chem. 280, 39175–39184 [DOI] [PubMed] [Google Scholar]
- 21. Tomas A., Meda P., Regazzi R., Pessin J. E., Halban P. A. (2008) Traffic 9, 813–832 [DOI] [PubMed] [Google Scholar]
- 22. Hata Y., Südhof T. C. (1995) J. Biol. Chem. 270, 13022–13028 [DOI] [PubMed] [Google Scholar]
- 23. Tellam J. T., Macaulay S. L., McIntosh S., Hewish D. R., Ward C. W., James D. E. (1997) J. Biol. Chem. 272, 6179–6186 [DOI] [PubMed] [Google Scholar]
- 24. de Wit H., Walter A. M., Milosevic I., Gulyás-Kovács A., Riedel D., Sørensen J. B., Verhage M. (2009) Cell 138, 935–946 [DOI] [PubMed] [Google Scholar]
- 25. Hammarlund M., Palfreyman M. T., Watanabe S., Olsen S., Jorgensen E. M. (2007) PLoS Biol. 5, e198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Torii S., Takeuchi T., Nagamatsu S., Izumi T. (2004) J. Biol. Chem. 279, 22532–22538 [DOI] [PubMed] [Google Scholar]
- 27. de Wit H., Cornelisse L. N., Toonen R. F., Verhage M. (2006) PloS One 1, e126 17205130 [Google Scholar]
- 28. Davletov B., Bajohrs M., Binz T. (2005) Trends Neurosci. 28, 446–452 [DOI] [PubMed] [Google Scholar]
- 29. Yu M., Kasai K., Nagashima K., Torii S., Yokota-Hashimoto H., Okamoto K., Takeuchi T., Gomi H., Izumi T. (2007) Mol. Biol. Cell 18, 688–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oh E., Spurlin B. A., Pessin J. E., Thurmond D. C. (2005) Diabetes 54, 638–647 [DOI] [PubMed] [Google Scholar]
- 31. Oh E., Thurmond D. C. (2009) Diabetes 58, 1165–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ohara-Imaizumi M., Nishiwaki C., Kikuta T., Nagai S., Nakamichi Y., Nagamatsu S. (2004) Biochem. J. 381, 13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]