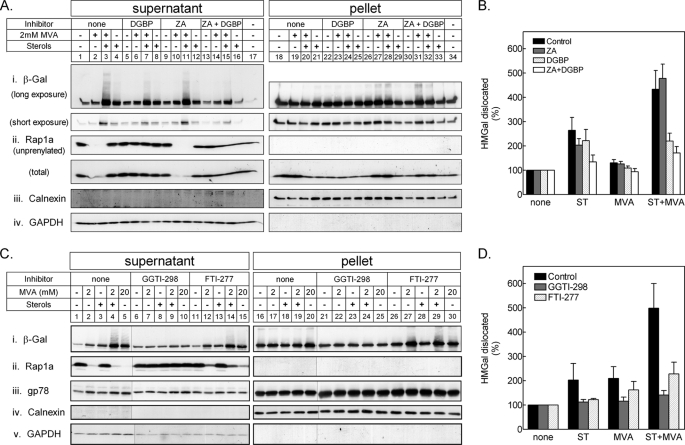
FIGURE 4.
Dislocation of HMGal requires protein geranylgeranylation. A, UT-2/HMGal cells were incubated for 20 h in Medium B supplemented with 50 μm lovastatin. The cells were then treated with ZA, DGBP, or both for 1 h before addition of 65 μm ALLN, sterols, and/or MVA, as indicated. Following an additional 7-h incubation, the cells were permeabilized in Solution C and fractionated by centrifugation, as described under “Experimental Procedures.” Aliquots of the supernatant and pellet fractions were immunoblotted with the indicated antibodies. Unprenylated and total Rap1a was detected with goat (SC-1482) and rabbit (SC-65) antibodies, respectively. B, densitometric analysis of the blots. For each inhibitor, the β-galactosidase immunoreactive signal in A, i (including trailing smear), was divided by the sum of β-galactosidase immunoreactive signals in pellet and supernatant, setting the value as “100%” for cells not treated with either sterols or MVA. Results are the mean ± S.E. of 8 independent experiments. C, UT-2/HMGal cells were set up as in A. Cells were treated for 1 h with either 50 μm GGTI-298 or 50 μm FTI-277 before an additional 3-h incubation with 65 μm ALLN and sterols, 2 mm MVA, or 20 mm MVA, as indicated. Cells were permeabilized and fractionated, as described. Aliquots of supernatant and pellet fractions were immunoblotted with the indicated antibodies. D, densitometric analysis of the blots as in B, except treatment with 20 mm MVA was not quantified. Results are the mean ± S.E. of 3 independent experiments.