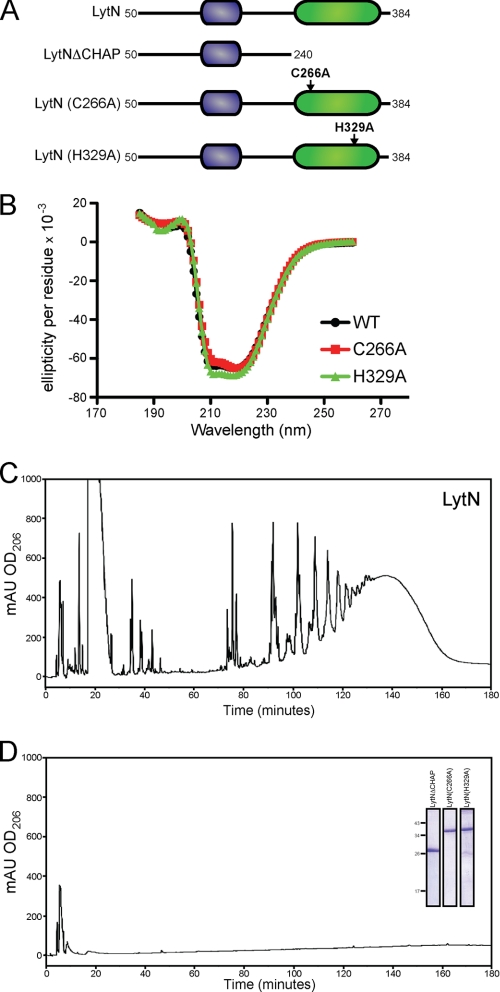
FIGURE 9.
CHAP domain of LytN is required for hydrolytic activity. A, diagram of the primary structure of rLytN constructs in which the CHAP domain is removed or specific amino acid substitutions have been introduced. Mutated amino acids are indicated with arrows. B, circular dichroism spectra of rLytN or its C266A and H329A substitution variants were analyzed in the far-UV range. C, rpHPLC of soluble rLytN-treated muropeptides. D, rpHPLC of muropeptides generated upon incubation of peptidoglycan with rLytNΔCHAP. The C266A and H329A variants also failed to cleave staphylococcal peptidoglycan. Inset, Coomassie Brilliant Blue-stained SDS-PAGE samples of purified rLytNΔCHAP, rLytNC266A, and rLytNH329A. mAU, milliabsorbance units.