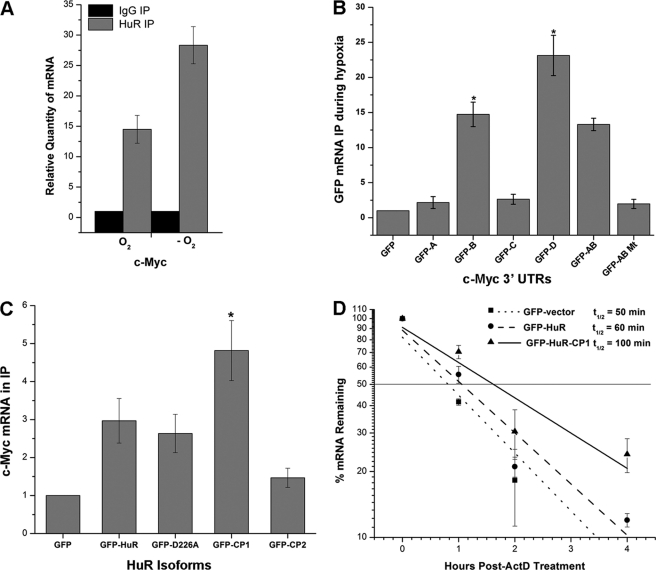
FIGURE 4.
HuR associates with c-myc mRNA during CoCl2-induced hypoxic stress. A, UM74B cells were grown with and without treatment of CoCl2 (O2 indicates no treatment, and −O2 represents CoCl2 treatment) and subjected to RNP IP followed by RT-qPCR analysis to measure the relative quantity of c-myc mRNA in HuR IP compared with control IgG IP. GAPDH serves as a loading control. B, shown are plasmids expressing chimeric RNAs spanning all the four c-myc 3′-UTR segments (A–D) combination plasmid vectors, AB and AB mutant (“Experimental Procedures”; see supplemental Fig. S2). 48 h after transfection, binding of HuR to each chimeric RNA was tested by RNP IP followed by GFP mRNA detection by RT-qPCR. C, forty-eight hours after transfection with a control plasmid, GFP or a plasmid overexpressing full-length HuR, HuR-D226A, HuR-CP1, and HuR-CP2 (“Experimental Procedures,” supplemental Fig. S3) c-Myc was measured after RNP IP using GFP antibody (loading control GAPDH from input lysates). D, treatment (48 h) with plasmids expressing GFP, GFP-HuR, and GFP-HuR-CP1 followed by the decay rates of c-myc was assessed in UM74B cells by RT-qPCR after transcription inhibition with actinomycin D (ActD). Values were normalized using a factor calculated from GAPDH gene expression. Error bars denote the S.E. of three sets of experiments (*, p < 0.05, n = 3).