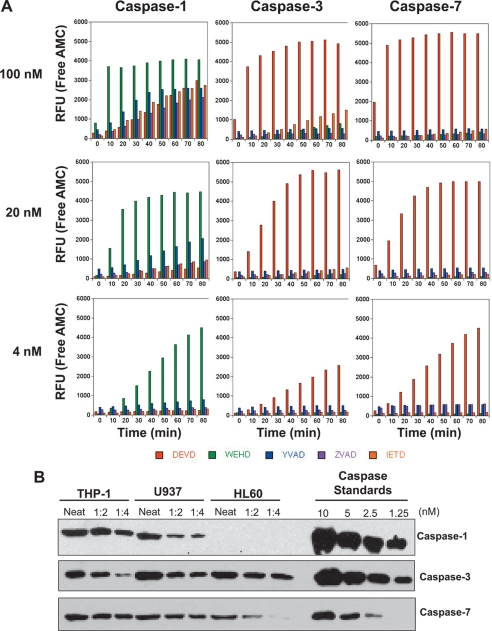
FIGURE 3.
Caspase-1 exhibits greater promiscuity toward synthetic substrates than caspases-3 or -7. A, hydrolysis of synthetic tetrapeptide substrates (DEVD-AMC, WEHD-AMC, YVAD-AMC, ZVAD-AMC, and IETD-AMC) by recombinant caspase-1, -3, or -7 at the indicated enzyme concentrations. B, Relative amounts of endogenous procaspase-1, -3, and -7 in the myelomonocytic cell lines, THP-1, U937, and HL-60. Cell lysates were generated from identical numbers of cells and were ran in a dilution series, as indicated, along with known amounts of the indicated recombinant procaspases. Caspases were detected by SDS-PAGE followed by immunoblotting. Results are representative of at least three independent experiments. RFU, relative fluorescence units.