FIGURE 5.
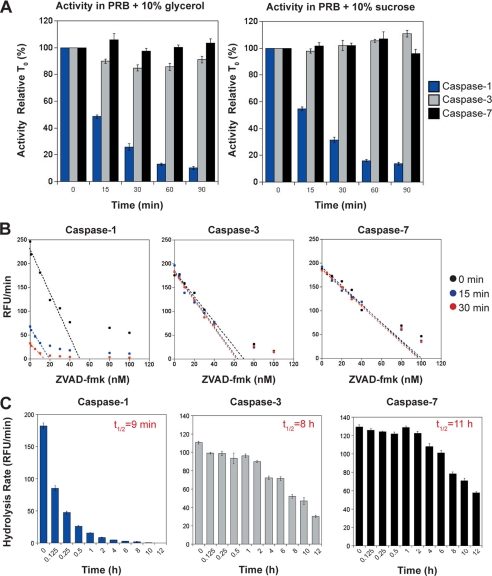
Processed caspase-1 is highly unstable compared with caspases-3 and -7. A, stability of caspase-1, -3, and -7 (all at 10 nm) at 37 °C under two different buffer conditions. Based on previous optimization studies, protease reaction buffer (PRB) was made to an optimal pH of 7.5 with the inclusion of two alternative stabilizers 10% glycerol or 10% sucrose, as indicated. B, active site titration of caspase-1, -3, and -7 following incubation at 37 °C for 0, 15, and 30 min. Line of best fit extrapolates the concentration of active enzyme remaining under each condition. The estimated concentration of active sites for caspases-3 and -7 was consistent under all conditions (for caspase-3, 0 min, 69 nm; 15 min, 67 nm; 30 min, 62 nm; for caspase-7, 0 min, 99 nm; 15 min, 98 nm; 30 min, 98 nm). However, the measured concentration of caspase-1 dropped by 63% (from 51 to 19 nm) after 15 min of preincubation at 37 °C, and by 73% (from 51 to 14 nm) after 30 min of preincubation 37 °C. C, long term stability of caspase-1, -3, and -7 (at 10 nm initial concentrations). Half-lives of caspase-1 (9 min), caspase-3 (8 h), and caspase-7 (11 h) were estimated by measuring the residual enzyme activity, by comparison with the initial input amounts, after incubation for the indicated times at 37 °C. Results shown represent the mean ± S.E. of three independent experiments. RFU, relative fluorescence units.