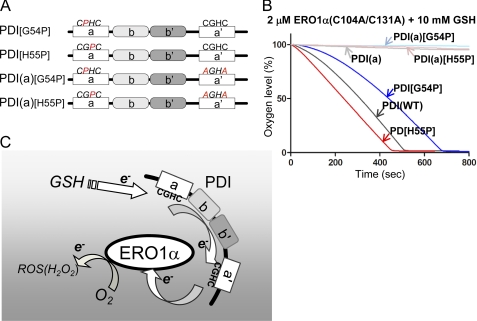
FIGURE 6.
Modulation of the reduction potential of the a domain affects the net oxidation rate of PDI by ERO1α. A, schematic representation of human PDI proteins with the mutated -CPHC-, -CGPC-, and -AGHA- sites indicated. B, the kinetics of oxygen consumption by 2 μm constitutively active ERO1α in a reaction containing 5 μm of each human PDI variant, as indicated in the figure, in the presence of GSH (10 mm). C, schematic model illustrating the interaction of and electron transfer relays between ERO1α and PDI. GSH supplies the electron, probably to the a domain of PDI. Following internal electron transfer from the a to the a′ domain, ERO1α receives the electron from the a′ domain. Intermolecular electron transfer would be stochastically constrained.