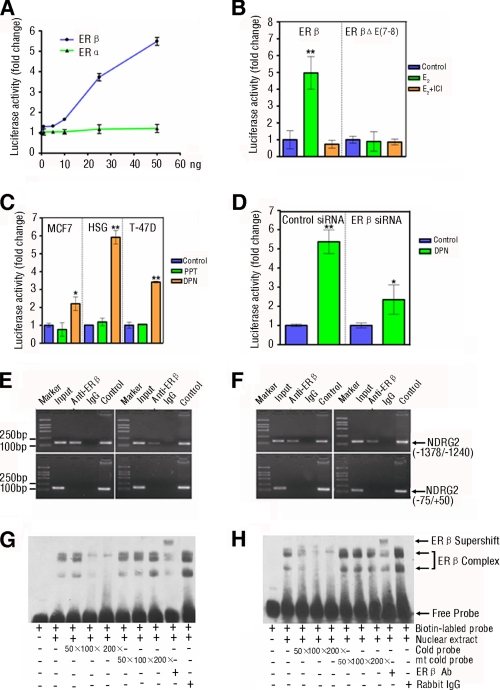
FIGURE 2.
ERβ mediates the estrogen-induced NDRG2 transcriptional activation by binding to the ERE of NDRG2 promoter in vivo and in vitro. A, ERβ, but not ERα, mediates the estrogen-induced NDRG2 transcriptional activation. HEK293 cells were co-transfected with NDRG2 promoter and increasing amounts of the expression vectors for human WT ERα or ERβ for 48 h, and then the cells were treated with 10 nm E2 for 12 h. The luciferase activity was measured. B, mutation in the ligand binding domain of ERβ loses the function. HEK293 cells were co-transfected with NDRG2 promoter and WT ERβ or the mutant with a deletion in ligand binding domain (ERβΔE(7–8)) for 48 h and treated with 10 nm E2 for 12 h in the presence or absence of 10 nm ICI 182,780 (ER antagonist). The luciferase activity was measured. C, DPN, but not PPT, enhances the NDRG2 transcriptional activation. MCF7, HSG, and T-47D cells that express endogenous ERα and ERβ were transfected with the NDRG2 promoter for 48 h and treated with ERα- or ERβ-selective agonist, 10 nm PPT or 10 nm DPN, or control ethanol for 12 h. The luciferase activity of individual groups of cells was measured. D, silence of ERβ expression reduces their responses to DPN. HSG cells were co-transfected with the NDRG2 promoter and 100 pmol of ERβ-specific siRNA or control siRNA for 48 h and treated with DPN for 12 h. The luciferase activity of individual groups of cells was measured. B–D, above experiments were performed in triplicate. Data are expressed as the means ± S.D. of relative changes of individual groups of cells to the control cells. *, p < 0.05; **, p < 0.01 versus control. E and F, binding of exogenous (left panel) or endogenous (right panel) ERβ to the ERE in the NDRG2 promoter in vivo was measured by ChIP assay. HSG (E) and HeLa (F) cells were transfected with expression vector for human ERβ or control vector for 48 h and treated with 10 nm E2 for 12 h. Subsequently, cell lysates were immunoprecipitated with anti-ERβ-specific antibody and analyzed by PCR amplification using the primers flanking the ERE in the NDRG2 promoter (−1378/−1240) or the control region (−75/+50), as indicated in supplemental Table S1. NDRG2 promoter plasmid and input chromatin (the remaining portion of cell lysates) were used as positive controls. ERβ antibody isotype rabbit IgG was used as negative control. G and H, binding of exogenous (G) or endogenous ERβ (H) to the ERE in NDRG2 promoter in vitro was measured by EMSA. HSG cells were transfected with the expression vector for human ERβ or control vector for 48 h and treated with 10 nm E2 for 12 h. Their nuclei were extracted and incubated with biotinylated WT probe for NDRG2-ERE in the presence or absence of increasing amounts of competitive unlabeled cold probes for NDRG2-ERE (WT or mutant) or the ERβ-specific antibody, respectively. The DNA-protein complexes were separated on a 6% TBE gel, transferred to nylon membranes, and visualized using peroxidase-conjugated streptavidin and substrate. Data shown are representative images from two repeated experiments.