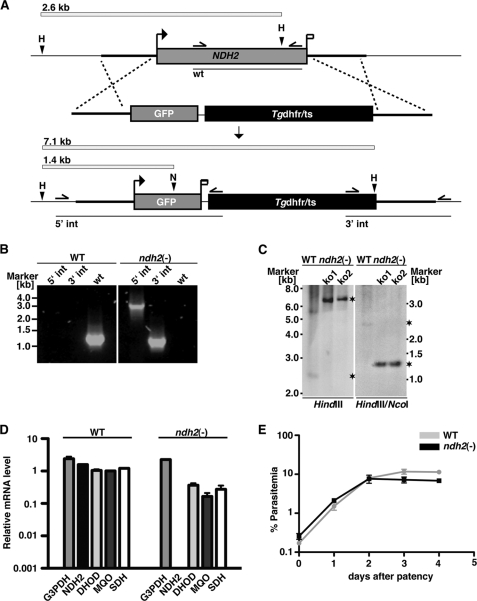
FIGURE 3.
P. berghei NDH2 is dispensable for asexual blood stages. A, the wild-type NDH2 locus is targeted with a linearized replacement plasmid containing the 5′- and 3′-UTRs of PbNDH2, GFP, and the positive selection marker Tgdhfr/ts. After double crossover homologous recombination, the NDH2 open reading frame is substituted by GFP and the selection marker, resulting in the loss-of-function ndh2(−) allele. GFP is now expressed under the PbNDH2 promoter and, therefore, indicates PbNDH2 promoter activity. Replacement- and WT-specific test primer combinations, expected PCR fragments, and predicted sizes of restriction endonuclease fragments are shown as arrows, lines, and white bars, respectively. H, HindIII; N, NcoI. B, confirmation of the NDH2 gene disruption by replacement-specific PCR analysis with primer combinations that amplify a signal in the recombinant locus (5′ int and 3′ int) only. The absence of a WT-specific signal in the clonal ndh2(−) population confirms the purity of the mutant parasite line. C, a Southern blot confirms the desired NDH2 deletion in two clones from two independent transfections (ko1 and ko2). Fragments are marked with asterisks. Restriction sites used for the digest of genomic DNA are indicated at the bottom. The 5′ flank of the targeting vector was used as probe for the Southern blot. D, quantitative RT-PCR from WT and ndh2(−) mixed blood stages is shown. Shown are transcript levels for glycerol-3-phosphate dehydrogenase (G3PDH; gi:68071805), NDH2, dihydroxyorotate dehydrogenase (DHOD; gi:68074653), malate:quinone oxidoreductase (MQO; gi:68075787), and succinate dehydrogenase (SDH; gi:68063151). Data were normalized to the putative aspartyl-tRNA synthetase (PBANKA_021020). Note the depletion of NDH2 transcripts in ndh2(−) parasites. E, ndh2(−) parasites cause high level parasitemia in vivo. Displayed are in vivo growth curves of WT (gray) and knock-out parasites (black). Animals (n = 3) were injected intravenously with 1,000,000 asexual parasites of the respective parasite populations. Parasitemia was determined every 24 h after infection by microscopic examination of Giemsa-stained blood smears.