FIGURE 4.
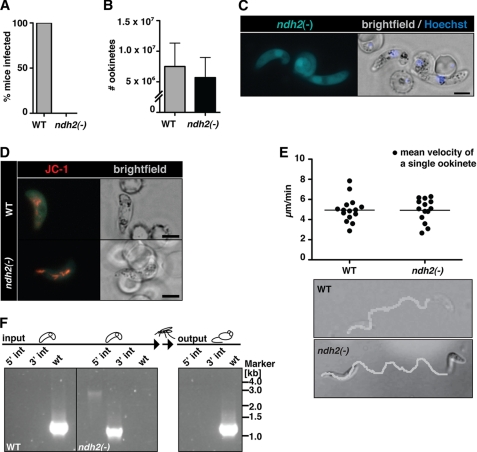
Ablation of NDH2 does not affect host switch from mammals to the insect vector. A, shown is a Plasmodium transmission experiment. Naïve mice were exposed to WT- or ndh2(−)-infected A. stephensi mosquitoes and examined daily for a blood stage infection. Data are from 3 separate experiments, with 2 ndh2(−) clones generated through separate transfection experiments and a total of 10 recipient mice. B, ookinete formation is not affected by the absence of NDH2. Ookinetes were formed in vitro in culture medium and quantified. Data are based on four in vitro cultures for each WT and ndh2(−). C, shown is epifluorescence live microscopy of a ndh2(−) ookinete. GFP expression confirms activity of the NDH2 promoter during sexual differentiation, ookinete viability, and successful integration of GFP into the NDH2 locus. Bar, 5 μm. D, mitochondrial membrane potential is detectable in WT and ndh2(−) ookinetes. Ookinetes were stained with the live stain JC-1, which forms red fluorescent aggregates in mitochondria if the membrane potential is intact. Bar, 5 μm. E, ookinete velocity is not affected by ablation of NDH2. Ookinetes in Matrigel were filmed for ∼10 min, and their tracks were quantified. Representative images of an ndh2(−) and a WT ookinete track (bottom) and mean velocity of WT ookinetes (n = 15) and ndh2(−) ookinetes (n = 14) (top) are shown. Note that all ndh2(−) ookinetes displayed gliding locomotion (n = 43).3 F, inability of ndh2(−) parasites to complete the life cycle is shown. A mix of both WT and KO ookinetes was membrane-fed to mosquitoes. After bite back, only WT blood stages could be detected in mice. Integration of KO construct and the absence of WT was monitored with PCR on genomic DNA derived from blood stage infection before setting up and mixing ookinete cultures (input) and after bite back (output).