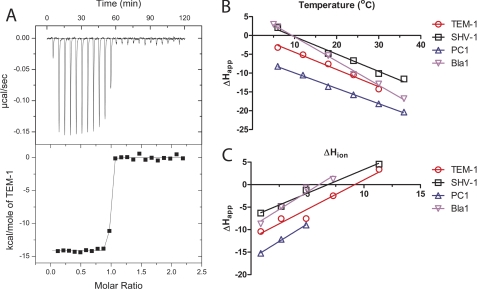
FIGURE 3.
ITC measurements of binding between BLIP-II and class A β-lactamases. A representative titration of the BLIP-II interactions is shown (A). In this particular titration, TEM-1 is injected into BLIP-II in phosphate-buffered saline at 30 °C. The steep transition region does not allow for an accurate measurement of affinity. B, the ΔCp of each interaction is calculated based on the slope of the line fitted to the apparent enthalpy (ΔHapp) shown at different temperatures (see “Experimental Procedures,” Equation 6). C, the protonation effect is shown as the ΔHapp varies depending on the buffer despite the same temperature (23 °C). The ΔHapp is plotted against the enthalpy of buffer ionization (ΔHion) to determine the number of protons absorbed upon complex formation (n) and the real binding enthalpy (ΔHreal) (see “Experimental Procedures,” Equation 7). Keys showing the colors corresponding to the respective β-lactamases are shown next to panels B and C.