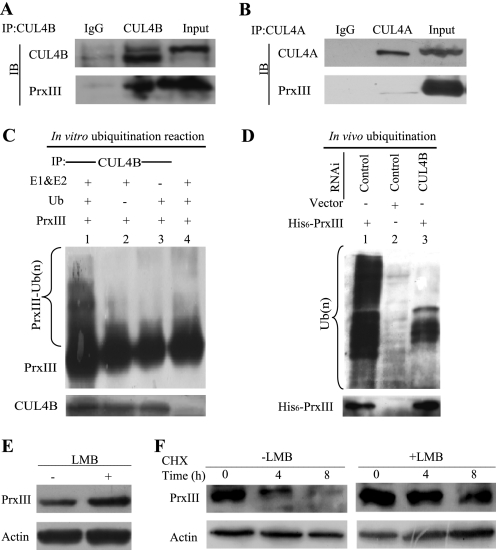
FIGURE 4.
CUL4B promotes polyubiquitination of PrxIII in vitro and in vivo. A, PrxIII interacts with the CUL4B complex in HEK293 cells. B, no PrxIII was detected in anti-CUL4A immunoprecipitates. Whole cell lysates were immunoprecipitated (IP) with the antibodies against the indicated proteins. Immunocomplexes were then subjected to immunoblotting (IB) using antibodies against the indicated proteins. C, in vitro ubiquitination of PrxIII by the CUL4B immunocomplex. For in vitro ubiquitination of PrxIII protein, a nickel column was used to purify His6-PrxIII from HEK293T cells, and the CUL4B immunocomplex and E3 were used as substrates, respectively. Assays were performed in 50-μl volume reactions containing each component, as indicated under “Experimental Procedures.” The reaction was conducted at 37 °C for 60 min, and the analysis was performed by immunoblotting with antibodies specific for PrxIII or CUL4B, as previously indicated. D, cell lysates were immunoprecipitated with an anti-His6 antibody and immunoblotted with an anti-Ub antibody to detect ubiquitylated PrxIII protein. E, LMB stabilizes PrxIII. HEK293 cells were transfected with negative control plasmids. After 24 h, the cells were either untreated or exposed to LMB for 6 h, and protein extracts were prepared. Equivalent amounts (30 μg) of the whole cell lysates were separated by SDS-PAGE and analyzed by immunoblotting with antibodies specific for the indicated proteins. F, CHX chase analysis of PrxIII protein degradation in LMB-treated and control cells. Protein levels at the indicated time points after adding CHX (at 50 μg/ml) to cells were analyzed by immunoblotting with antibodies specific for the indicated proteins.