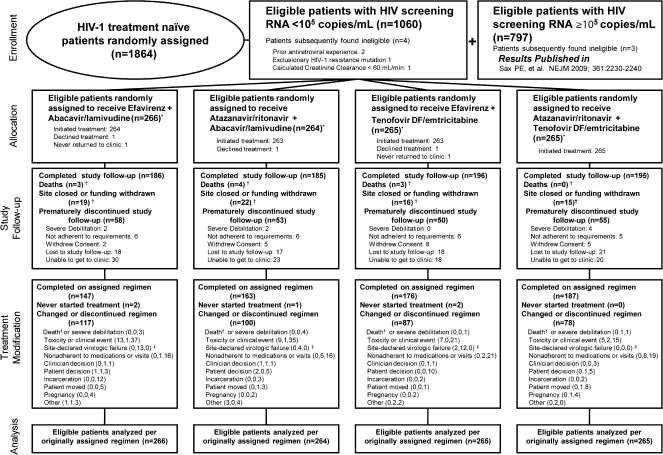
Figure 1.
Enrollment, randomization, and disposition of patients with screening HIV RNA <105 copies/mL.
Patients were to remain in follow-up regardless of whether antiretroviral therapy was modified; therefore, study follow-up and treatment modification disposition are both presented. Reasons for treatment modification are split into (number before, number after, number without protocol-defined virologic failure) to summarize the amount of censoring of primary efficacy endpoints in as-treated analyses. * Nucleoside reverse transcriptase inhibitors were blinded through 25 February 2008 for persons with HIV-1 RNA levels of 100 000 copies/mL or more at screening and until final visits starting 1 July 2009 for those with HIV-1 RNA levels less than 100 000 copies/mL at screening. † Death was censored for premature study discontinuation and counted as a reason for treatment discontinuation if there was no previous regimen modification. Site closure was censored for premature study and treatment discontinuation. ‡ Site-declared virologic failure was by clinical determination of the site investigator, whereas protocol-defined virologic failure was determined strictly by the quantitative definition set forth in the protocol. Numbers may differ because not all patients who had protocol-defined virologic failure modified the regimen, or the drug modification may have been attributed to another reason, such as “nonadherent with medications or visits.”