Table 2.
Substrate scope
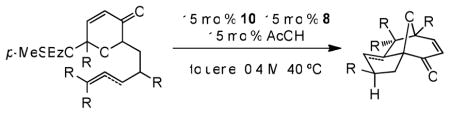 | |||||
|---|---|---|---|---|---|
| entry | substrate | product | time (h) | yield (%)a | ee (%)b |
| 1c,d |
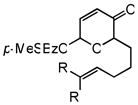 5c |
 6 |
48 | 74 | 91 |
| 2 |
11 R=Me R′=H |
12 | 72 | 70 | 90 |
| 3 |
13 R=H R′=Me |
14 | 72 | 66 | 89 |
| 4 |
15 R=Me R′=Me |
16 | 96 | 51 | 89 |
| 5 |
17 R=H R′=Ph |
18 | 72 | 48 | 86 |
| 6 |
19 R=CO2Et R′=H |
20 | 72 | 66 | 90 |
| 7e |
21 R=CO2Me R′=Me |
22 | 96 | 37 | 80 |
| 8c,d |
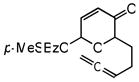 23 |
 24 |
72 | 54 | 95 |
| 9 |
 25 |
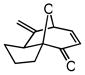 26 |
72 | 42 | 88 |
| 10d |
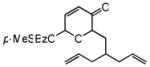 27 |
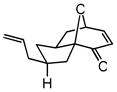 28 |
72 | 77 | 90 |
| 11 |
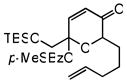 29 |
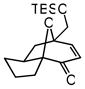 30 |
72 | 70f | 89f |
Isolated yields after chromatography on silica gel.
Determined by HPLC using commercial chiral columns.
10 mol% 10 + 8.
The absolute stereochemistry of 24 and derivatives of 28 and 6 were determined by Xray crystallography and that of all other products was assigned by analogy.
20 mol% 10 + 8.
Determined on the free alcohol.
