Fig. 3.
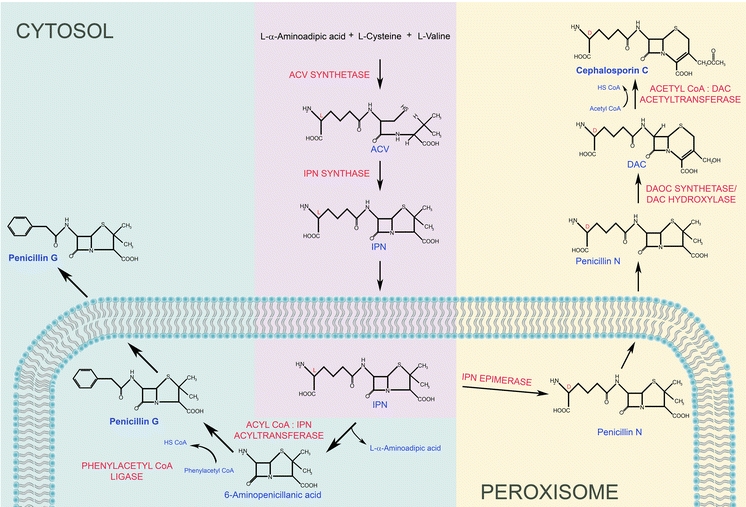
Hypothetical model of the compartmentalization of biosynthetic pathways involved in penicillin and cephalosporin C formation. The fungal β-lactam antibiotics derive from three amino acids (l-α-aminoadipic acid (l-α-AAA), l-cysteine, and l-valine, which are condensed via the function of ACV synthetase) into the tripeptide δ-(l-α-aminoadipyl)-l-cysteinyl-d-valine (ACV) in the cytosol. Subsequently the bicyclic ring structure IPN is formed via oxidative ring closure from the linear ACV tripeptide by cytosolic IPN synthase. IPN is subsequently transported into the peroxisome by the CefP transporter. The penicillin and cephalosporin biosynthesis pathways diverge at this stage. Penicillin biosynthesis occurs in peroxisomes where the hydrophilic l-α-AAA side chain of IPN is exchanged for a hydrophobic acyl group of a side chain precursor by acyl coenzyme A (CoA):isopenicillin N acyltransferase (IAT). In the natural environment penicillin DF, F and K, containing hexenoic acid, ∆3-hexenoic acid and octenoic acid as a side chain, respectively, are produced. Penicillin G and V are synthesized upon supplementation of cultivation media with PAA or POA, respectively. Activation of the side chain precursor in the peroxisomal matrix requires phenylacetyl coenzyme A ligase (PCL) (Koetsier et al. 2009; Meijer et al. 2010). In A. chrysogenum, two enzymes are employed in the subsequent step of the cephalosporin biosynthesis pathway, IPN–CoA synthetase, IPN–CoA epimerase. These enzymes are the components of the IPN epimerase system and catalyse the isomerization of the l-α-AAA side chain of IPN to the d-enantiomer to produce penicillin N. Bioinformatic analysis of genes encoding two components of the IPN epimerase system, cefD1 and cefD2, revealed that both proteins contain putative peroxisomal targeting signals (Martin et al.). In P. chrysogenum the putative homologous proteins of the IPN epimerase system were found in the peroxisomal matrix supporting the hypothesis that this step of cephalosporin biosynthesis may occur in peroxisomes (Kiel et al. 2009). Moreover, recent studies have revealed the presence of two peroxisomal membrane proteins: CefP and CefM, implied for the translocation of the penicillin N intermediate. The completion of cephalosporin biosynthesis occurs in the cytosol, where bifunctional enzyme with expandase and hydroxylase activities converts penicillin N into deacetoxycephalosporin C and subsequently into deacetylcephalosporin C (DAC). The thiazolidine ring of penicillin is exchanged by the six-membered dihydrothiazine ring forming the cephem nucleus. In the final step, which is catalyzed by acetyl-CoA:DAC acetyltransferase (DAT), DAC is converted into cephalosporin C
