Summary
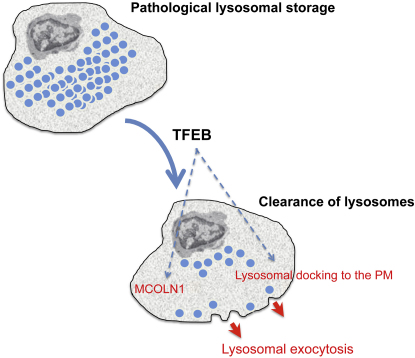
Lysosomes are cellular organelles primarily involved in degradation and recycling processes. During lysosomal exocytosis, a Ca2+-regulated process, lysosomes are docked to the cell surface and fuse with the plasma membrane (PM), emptying their content outside the cell. This process has an important role in secretion and PM repair. Here we show that the transcription factor EB (TFEB) regulates lysosomal exocytosis. TFEB increases the pool of lysosomes in the proximity of the PM and promotes their fusion with PM by raising intracellular Ca2+ levels through the activation of the lysosomal Ca2+ channel MCOLN1. Induction of lysosomal exocytosis by TFEB overexpression rescued pathologic storage and restored normal cellular morphology both in vitro and in vivo in lysosomal storage diseases (LSDs). Our data indicate that lysosomal exocytosis may directly modulate cellular clearance and suggest an alternative therapeutic strategy for disorders associated with intracellular storage.
Graphical Abstract
Highlights
► TFEB-regulated transcription induces lysosomal docking to the plasma membrane (PM) ► TFEB promotes lysosomal fusion with the PM by raising Ca2+ levels through MCOLN1 ► TFEB can thus rescue pathological storage in lysosomal storage disease (LSD) cells ► In vivo TFEB gene delivery rescues storage, inflammation, and apoptosis in LSD mice
Introduction
Lysosomes are cellular organelles primarily involved in degradation and recycling processes (Kornfeld and Mellman, 1989). Lysosomes are also involved in a secretory pathway known as lysosomal exocytosis, which requires two sequential steps. In the first step, Ca2+-independent (Jaiswal et al., 2002), lysosomes are recruited to the close proximity of the cell surface, while in the second step the pool of predocked lysosomes fuse with the plasma membrane (PM) in response to Ca2+ elevation (Andrews, 2000; Jaiswal et al., 2002; Tucker et al., 2004). Lysosomal exocytosis plays a major role in several physiological processes such as cellular immune response, bone resorption, and PM repair (Andrews, 2000, 2005; Bossi and Griffiths, 2005). Ca2+-dependent lysosomal exocytosis was considered to be limited to specialized secretory cells; however, recent studies indicate that this process occurs in all cell types (Andrews, 2000; Rodríguez et al., 1999; Rodríguez et al., 1997). Although the main steps of lysosomal exocytosis have been elucidated, little is known about its regulation and how this process is coordinated with lysosomal biogenesis.
We recently discovered that lysosomal biogenesis and lysosomal degradative function are transcriptionally regulated by the bHLH-leucine zipper transcription factor EB (TFEB) (Sardiello et al., 2009). TFEB activation was able to reduce the accumulation of the pathogenic protein in a cellular model of Huntington disease (Sardiello et al., 2009) and ameliorated the phenotype of cells from a murine model of Parkinson disease (Dehay et al., 2010).
In this study we demonstrated that TFEB transcriptionally regulates lysosomal exocytosis both by inducing the release of intracellular Ca2+ through its target gene MCOLN1 and by increasing the population of lysosomes ready to fuse with the PM. Moreover, we demonstrated that the induction of lysosomal exocytosis by TFEB promotes cellular clearance in pathological conditions such as lysosomal storage diseases (LSDs) in which the lysosomal degradative capacity of cells is compromised.
Results and Discussion
TFEB Overexpression Induces Lysosomal Exocytosis
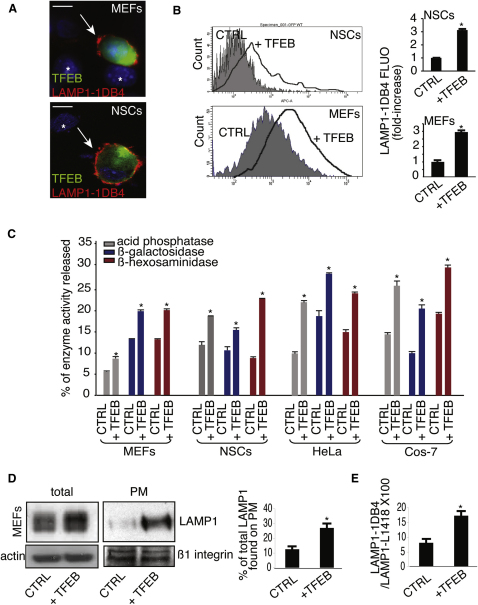
A typical hallmark of lysosomal exocytosis is the translocation of lysosomal membrane markers to the PM (Reddy et al., 2001; Rodríguez et al., 1997; Yogalingam et al., 2008). TFEB overexpression in mouse embryonic fibroblasts (MEFs) and neuronal stem cells (NSCs) resulted in an increased exposure of the luminal domain of LAMP1, a lysosomal membrane marker, on the PM (Figure 1A). Consistently, quantitative analysis by flow cytometry (FACs) showed an increase of LAMP1 staining on the PM of TFEB-overexpressing cells (Figure 1B). A direct consequence of lysosomal exocytosis is the release of lysosomal enzymes into the cell culture medium (Rodríguez et al., 1997). Significantly higher levels of lysosomal hydrolases were detected in the medium of several cells lines overexpressing TFEB compared with control cells (Figure 1C). The increase of lysosomal enzymes in the medium was not associated with an increase in the levels of cytosolic lactate dehydrogenase (LDH), thus excluding that the release of lysosomal enzymes was due to cell damage (see Figure S1A available online). Together, these data indicate that TFEB induces lysosomal exocytosis.
Figure 1.
TFEB Overexpression Induces Lysosomal Exocytosis
(A) Confocal microscopy images showing the exposure of LAMP1 on the PM in both nonpermeabilized NSC cells and MEFs transfected with either a bicystronic plasmid expressing TFEB-GFP or with an empty vector. LAMP1 was detected using an antibody against its luminal portion (LAMP1-1DB4). TFEB-transfected cells were localized by the expression of GFP; nontransfected cells are indicated by asterisks.
(B) Quantitative analysis by flow cytometry of LAMP1 levels on the PM in both NSCs and MEFs that express either a bi-cystronic TFEB-GFP plasmid or GFP. Bars represent the fold increase of LAMP1 fluorescence on PM in TFEB-transfected versus GFP-transfected (control) cells.
(C) TFEB overexpression increases the release of lysosomal enzymes in the culture medium of MEFs, NSCs, HeLa, and COS-7 cells. Activities of lysosomal enzymes acid phosphatase, β-galactosidase, and β-hexosaminidase were determined in the culture medium and in cells transfected with either an empty vector or with a TFEB-expression vector. The figure shows percentages of enzyme activities released compared with total activities.
(D) Representative immunoblots showing LAMP1 levels in both total lysates and enriched PM extracts from MEFs transfected with either TFEB or with an empty vector. Results were normalized using an antibody against actin and the PM protein β1-integrin, respectively. The histogram shows the quantification of LAMP1 detected by the immunoblot.
(E) Total and surface LAMP1 levels after TFEB overexpression were analyzed by flow cytometry analyses using the LAMP1-1DB4 and the LAMP1-L1418 antibodies that are able to detect PM- and intracellular-LAMP1, respectively. Data represent mean ± SEM; ∗p < 0.05 (B, C, D, and E). Scale bar represents 10 μm (A).
Western blot and FACs analyses revealed an enrichment of LAMP1 on the PM compared with total LAMP1 in TFEB-overexpressing cells, suggesting that the elevation of LAMP1 on the PM was not a mere consequence of TFEB-mediated expansion of the lysosomal compartment and of the consequent increase of LAMP1 protein levels (Sardiello et al., 2009), but it reflected an active movement of lysosomes toward the PM (Figures 1D and 1E).
To exclude the possibility that TFEB-mediated induction of LAMP1 expression resulted in an overloading of the trans-Golgi network (TGN) with a consequent abnormal sorting of LAMP1 directly from the TGN to the PM, we performed experiments using the temperature-sensitive VSVG-GFP protein (Figure S1B). This approach is generally used to monitor transport through the TGN-to-PM segment of the secretory pathway (Matlin and Simons, 1984). At 20°C, both control and stable HeLa cells expressing TFEB (CF7 cells) exhibited the VSVG protein trapped in the Golgi area, while the staining for LAMP1 decorated a different compartment with a typical spotty lysosome-like pattern (Figure S1B, left panel arrows). This indicates that the exposure to the low temperature did not cause the accumulation of a detectable amount of newly synthesized LAMP1 within the Golgi, despite overexpression of TFEB. The temperature shift from 20°C to 37°C triggered VSVG exit from the Golgi toward the PM (Figure S1B, middle panel arrows), while again no VSVG overlap with LAMP1 was detected within intracellular structures directed toward the cell surface. Finally, blocking fusion of Golgi-derived VSVG transport carriers with the PM (Polishchuk et al., 2004; see also Experimental Procedures) by the use of 0.5% tannic acid (TA) resulted in the accumulation of VSVG transport carriers near the PM (Figure S1B, right panel arrows), as expected. However, no LAMP1 was detected within such VSVG-positive structures, suggesting that LAMP1 was not delivered to the PM from the Golgi in CF7 cells. These results clearly indicate that LAMP1 does not escape the lysosomal route in TFEB-overexpressing cells and support our conclusion that the increase of LAMP1 on the PM is due to enhanced lysosomal exocytosis.
TFEB Overexpression Enhances Lysosomal Predocking to the PM and Elevates Intracellular Ca2+
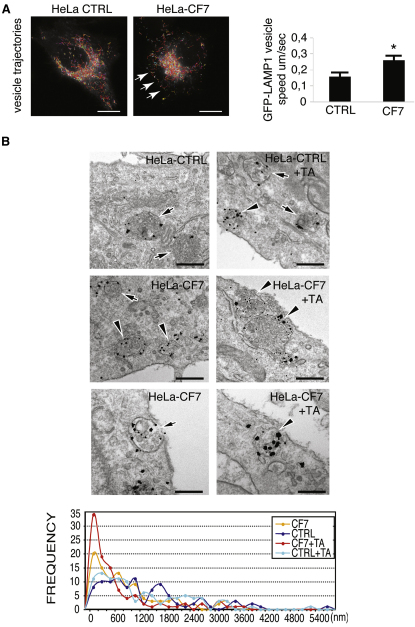
Induction of lysosomal exocytosis involves the recruitment of lysosomes to the PM (Blott and Griffiths, 2002). TFEB overexpression resulted in an increased motility of lysosomes (Figure 2A; Movies 1 and 2). The impact of TFEB overexpression on lysosomal recruitment was investigated using the immuno-EM approach. To this end, control HeLa and CF7 cells were labeled with an antibody against LAMP1 and prepared for ultrastructural analysis, as previously described (Polishchuk et al., 2003). In thin sections, LAMP1 was detected at vacuolar structures that contained multiple intralumenal vesicles and/or electron-dense material. These structures could be identified as lysosomes on the basis of their ultrastructural features (Figure 2B, arrows and arrowheads). In HeLa control cells, lysosomes were distributed randomly throughout the cells, often at a substantial distance from the cell surface (Figure 2B, upper left panel and arrows). To reveal the pool of lysosomes that undergo exocytosis, we incubated cells for 1 hr in the medium containing 0.5% TA, which is widely used as a tool to block fusion of the different exocytic organelles with the PM (Newman et al., 1996; Polishchuk et al., 2004). After the incubation with TA, the cells were fixed and processed for immuno-EM. Exposure of control HeLa cells to TA induced a moderate increase in LAMP1-positive structures located very close (within 200-400 nm) to the cell surface (Figure 2B, upper right panel, arrowhead and frequency plot), indicating that only a small proportion of the lysosomes underwent exocytosis over the treatment period. In contrast, CF7 cells exhibited numerous lysosomes in the close proximity of the PM (Figure 2B, middle and lower left panels, arrowheads, and frequency plot), even in the absence of TA. Block of fusion by TA further increased the pool of the LAMP1-positive structures near the cell surface, with many of them being almost attached to the PM (Figure 2B, middle and lower right panels, arrowheads, and frequency plot). It is important to note that the vast majority of such LAMP1 structures in TA-treated CF7 cells resembled lysosomes rather than Golgi-derived vesicles, in terms of both size and ultrastructural features. Notably, the magnitude of such an increase in the frequency of PM-associated lysosomes upon the block of fusion was significantly higher in TFEB-overexpressing cells compared with the control HeLa line (Figure 2B, frequency plot).
Figure 2.
TFEB Enhances Trafficking and PM Proximity of Lysosomes
(A) TFEB enhances the movement of lysosomes to PM. Merged time-lapse frames of control and stable TFEB-overexpressing HeLa cells (HeLa-CF7) transfected with a LAMP1-GFP vector showing the trajectories of lysosomes. The mean velocity was calculated using IMAGEJ software (see Experimental Procedures for details).
(B) Immuno-EM reveals increase in lysosomal exocytosis upon overexpression of TFEB. Control and stable TFEB-overexpressing HeLa cells (HeLa-CF7) were fixed directly or 60 min after addition of 0.5% TA to the medium (see Experimental Procedures). Then cells were labeled with antibodies against LAMP1 and prepared for immuno-EM (see Supplemental Experimental Procedures). In control HeLa cells, lysosomes decorated by LAMP1 are often detected at a substantial distance from the cell surface (arrows), while treatment with TA only slightly increases the frequency of lysosomes that are close to the PM (arrowheads). TFEB-overexpressing cells (CF7) contain numerous lysosomes located near the cell surface (arrowheads), although several LAMP1-positive structures could be detected toward the cell interior as well (arrow). TA further induced accumulation of lysosomes in subsurface area of TFEB-overexpressing cells with many lysosomes almost touching the PM (arrows). The distance between lysosomes and PM was estimated in thin sections as described in Experimental Procedures in control and TFEB-overexpressing cells incubated with or without TA (100 lysosomes were counted for each condition). The number of lysosomes, as a function of their distance from the cell surface, was counted over 200 nm intervals (100 lysosomes were counted for each experimental condition). Scale bars represent 10 μm (A) and 350 nm (B). Data represent mean ± SEM; ∗p < 0.05 (A).
We further confirmed the latter results by loading control and TFEB-overexpressing HeLa cells with BSA gold (5 nm) for 30 min and chasing them in BSA gold–free medium for 120 min (Figure S2). In these conditions, all of the endosomal structures that present BSA gold are late endosomes/lysosomes (Parton et al., 1989). In CF7 cells, the distance between the endo/lysosomes structures and the PM was strongly reduced (mean distance HeLa control 2387nm versus 438nm in HeLa CF7) when the total number of endo/lysosomes (58 versus 73) was increased (Figure S2). Notably, fusion of lysosomes with the PM and release of BSA-stained lysosomes in the extracellular space were detected in TFEB-overexpressing cells (Figure S2, lower right panel). Thus, taken together, these findings indicate that TFEB overexpression significantly stimulates the recruitment of lysosomes to the PM, a step that is required for lysosomal exocytosis.
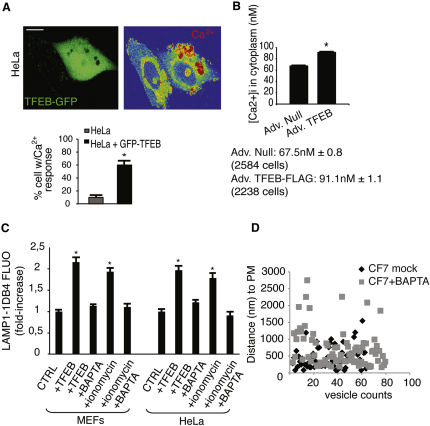
Previous studies demonstrated the requirement Ca2+ elevation for the fusion of lysosomes with the PM but not for the recruitment of lysosomes to the cell surface (Andrews, 2000; Jaiswal et al., 2002; Rodríguez et al., 1997). Confocal imaging showed an elevation of intracellular Ca2+ levels in TFEB-GFP-positive cells (Figure 3A), consistent with the role of TFEB in the induction of lysosomal exocytosis. To quantify the extent of TFEB-mediated Ca2+ elevation, we measured the cytoplasmic concentration of Ca2+ by a ratiometric assay (Kao et al., 2010). We observed a very significant increase in Ca2+ concentration in TFEB-overexpressing CF7 HeLa cells compared with control cells (91.4 nM ± 1.1 and 67.5 nM ± 0.8, respectively) (Figure 3B). Moreover, depletion of Ca2+ by the use of Ca2+ chelators in TFEB-overexpressing cells completely inhibited the appearance of LAMP1 on PM (Figure 3C). As expected, TFEB-mediated recruitment of lysosomes to the PM, as measured by EM analysis of the distance of the endo-lysosomes structures to the PM in BSA gold-loaded cells, was not affected by depletion of Ca2+ in CF7 cells (Figure 3D).
Figure 3.
TFEB-Mediated Induction of Lysosomal Exocytosis Is Associated with Intracellular Ca2+ Release
(A) Analysis of intracellular Ca2+ by confocal microscopy of HeLa cells transfected with a bi-cystronic TFEB-GFP construct. Data are displayed as the percentage of cells with Ca2+ response compared with the nontransfected cells.
(B) TFEB overexpression elevates cytosolic Ca2+ levels. HeLa cells were infected with either control adenovirus (Ad. Null) or adenovirus expressing TFEB (Ad. TFEB-FLAG) and loaded with Fura-2AM. Cytosolic Ca2+ concentration was calculated as described in Supplemental Experimental Procedures.
(C) TFEB-mediated lysosomal exocytosis is Ca2+-dependent. MEFs and HeLa cells transfected with either TFEB or an empty vector were treated with either the Ca2+ ionophore ionomycin (10 micromolar) or the membrane-permeable Ca2+ chelator BAPTA-AM (30 micromolar), or both. Mean values of cell surface LAMP1 fluorescence were quantified by flow cytometry.
(D) Ca2+ depletion in HeLa-overexpressing TFEB (HeLa CF7) does not affect endolysosomes' distance to the PM. HeLa q < CF7 loaded with BSA gold, following the same protocol described in (B), were treated with the Ca2+ chelator BAPTA-AM (30 micromolar) for 30 min before the fixation. Morphometric analysis was performed to visualize the distance of endo-lysosomes to the PM. Scale bar represents 10 μm (A). Data represent mean ± SEM; ∗p < 0.05 (A, B, and C).
Together, these results indicate that in addition to promoting lysosomal recruitment to the PM, TFEB induces lysosomal exocytosis by enhancing Ca2+-mediated fusion of lysosomes with the PM.
TFEB Releases Ca2+ from Acidic Stores through the Activation of Mucolipin-1
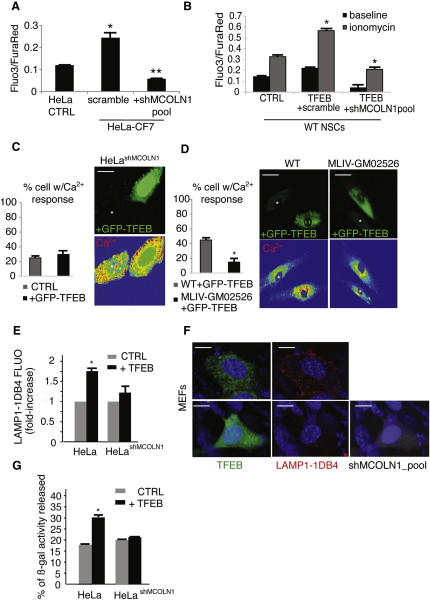
Mucolipin 1 (MCOLN1) is a lysosomal nonselective cation channel that is mutated in mucolipidosis IV (MLIV), a severe type of LSD (Bargal et al., 2000,2002; Bassi et al., 2000). Previous studies reported that lysosomal exocytosis is reduced in MLIV cells (LaPlante et al., 2006) and increased in cells expressing gain-of-function MCOLN1 mutations (Dong et al., 2009). These observations suggest that MCOLN1, upon a proper cellular stimulation, mediates intralysosomal Ca2+ release to trigger lysosomal exocytosis. In a separate study, we demonstrated that the gene encoding MCOLN1 is regulated by TFEB (Palmieri et al., 2011), making it an appealing candidate to mediate TFEB effects on lysosomal exocytosis. Remarkably, TFEB-mediated increase of intracellular Ca2+ was blocked in CF7 cells and in NSCs overexpressing TFEB by transient silencing of MCOLN1 with a pool of four fluorescently tagged vectors carrying specific MCOLN1-shRNAs (Figures 4A and 4B). Confocal Ca2+ imaging of HeLa cells stably depleted for MCOLN1 using a single shRNA vector (HeLashMCOLN1) failed to detect an elevation of intracellular Ca2+ in TFEB-overexpressing cells (Figure 4C). Consistently, Ca2+ levels were not affected by TFEB overexpression in human MLIV cells that carry loss of function mutations of MCOLN1 (Figure 4D). In addition, stable depletion of MCOLN1 in HeLa cells (Figure 4E) or transient depletion by a pool of shRNA vectors against MCOLN1 (Figure 4F) impaired TFEB-mediated fusion of LAMP1 with the PM, and inhibited the secretion of lysosomal enzymes into the medium (Figure 4G). Similar results were obtained by transient expression of individual shRNAs against MCOLN1 (Figure S3A). To exclude off-target effects of shRNA contructs, we performed a rescue experiment using a vector carrying an shRNA-resistant mutant form of MCOLN1 (MCOLN1 mut) (Vergarajauregui and Puertollano, 2006). Overexpression of MCOLN1 mut in HeLashMCOLN1 significantly recovered the appearance of surface of LAMP1 after TFEB overexpression (Figure S3B).
Figure 4.
TFEB Elevates Intracellular Ca2+ Levels through the Activation of MCOLN1
(A) Flow cytometric Ca2+ flux assay of stable TFEB-transfected HeLa (CF7) and control cells transfected with a vector containing a scramble shRNA or a specific shRNA against MCOLN1. Data represent the mean values of the Fluo3/FuraRed ± SD ratio.
(B) Flow-cytometry Ca2+ flux assay in NSCs transfected with a control vector (CTRL), a vector containing a scramble shRNA plus TFEB plasmid, and a vector containing a specific shRNA against MCOLN1 plus TFEB plasmid. Ca2+ was determined in resting condition and after stimulation with ionomycin (10 micromolar).
(C and D) Analyses of intracellular Ca2+ by confocal microscopy of (C) a stable HeLa clone expressing specific shRNA against MCOLN1 (HeLa shMCOLN1) and (D) human MLIV fibroblasts transfected with a bi-cystronic TFEB-GFP construct. Data are displayed as the percentage of cells with Ca2+ response compared with nontransfected cells.
(E) Flow-cytometry analysis of LAMP1 on the PM of HeLashMCOLN1 cells transfected with TFEB.
(F) Silencing of MCOLN1 reduces TFEB-mediated translocation of LAMP1 to the PM. Representative confocal microscopy images of nonpermeabilized wild-type MEFs transfected with either a bicystronic TFEB-GFP (green in the figure) or with a bi-cystronic TFEB-GFP vector. Cells were cotransfected with a vector containing a specific shRNA against MCOLN1 tagged with RFP (gray in the figure), and stained for luminal LAMP1 (LAMP1-1DB4; red in the figure).
(G) Secretion of lysosomal β-galactosidase in HeLashMCOLN1 cells. The displayed values represent secretion efficiency of the indicated enzyme, which was calculated as the % of enzymatic activity in the medium with respect to the total activity (medium and cellular pellet). Data represent mean ± SEM; ∗p < 0.05 (A–E and G). Scale bars represent 10 μm (C and F) and 25 μm (D).
The lysosomal localization of MCOLN1 and its channel properties suggest that the elevation of intracellular Ca2+ levels induced by TFEB overexpression is due to the release of Ca2+ from lysosomal stores through MCOLN1. To test this hypothesis, we measured cytosolic Ca2+ levels in TFEB-overexpressing cells by confocal imaging time-lapse after inducing acidic Ca2+ release with Bafilomycin A, an inhibitor of the vacuolar H+-ATPase, which is responsible for the proton gradient driving lysosomal Ca2+ uptake (Christensen et al., 2002). The results clearly showed a local increase of cytosolic Ca2+ levels in the stable HeLa-CF7 clone, in the transiently transfected TFEB-GFP overexpressing HeLa cells and in HeLa cells infected with an adenovirus encoding MCOLN1 (Figure S3C), compared with control HeLa cells. Conversely, HeLa cells stably depleted of MCOLN1 were not able to increase Ca2+ levels after TFEB-GFP overexpression (Figure S3C). Notably, both TFEB and MCOLN1 overexpressing cells were less sensitive to Bafilomycin A1-dependent depletion of lysosomal Ca2+ compared with HeLa control cells (Figure S3D). Together these data strongly suggest that MCOLN1-dependent release of Ca2+ from acidic Ca2+ stores plays a major role in TFEB-mediated lysosomal exocytosis.
TFEB-Mediated Lysosomal Exocytosis Promotes Cellular Clearance in Cellular Models of LSDs
LSDs are monogenic disorders characterized by the progressive accumulation of undegraded material in the lysosomes due to the deficiency of specific lysosomal proteins (Suzuki, 2002; Neufeld, 1979-1980). This is often associated with intracellular trafficking abnormalities and with a block of the autophagic pathway, leading to a progressive multisystemic phenotype that is often associated with neurodegeneration (Ballabio and Gieselmann, 2009; Fraldi et al., 2010; Futerman and van Meer, 2004; Platt and Walkley, 2004; Settembre et al., 2008). Interestingly, a previous study reported induction of lysosomal exocytosis in metachromatic leukodystrophy, a neurodegenerative form of LSD (Klein et al., 2005). We tested whether TFEB-induced lysosomal exocytosis could be exploited to induce cellular clearance in multiple sulfatase deficiency (MSD) and mucopolysaccharidosis type IIIA (MPS-IIIA), two different types of LSDs with a severe neurodegenerative outcome due to the progressive accumulation of glycosaminoglycans (GAGs) and severe vacuolization in postmitotic neurons and glial cells (Platt and Walkley, 2004; Futerman and van Meer, 2004).
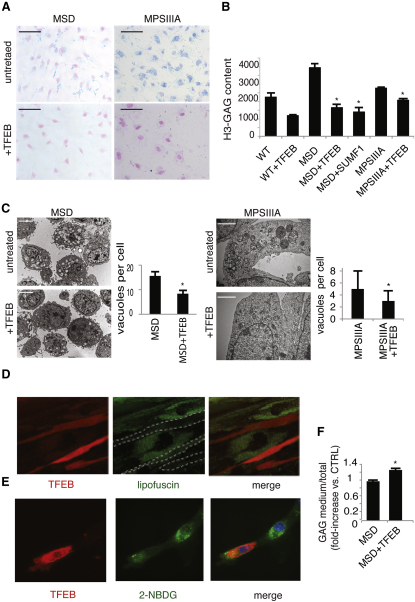
Overexpression of TFEB in MEFs derived from the murine models of MSD and MPSIIIA resulted in an even more significant increase of LAMP1 on the PM (Figure S4A) and of lysosomal enzymes into the culture medium (Figure S4B), compared with that observed in control cells overexpressing TFEB. This indicates that LSD cells efficiently respond to TFEB-mediated induction of lysosomal exocytosis. Therefore, we evaluated the effect of TFEB overexpression on the clearance of GAGs in glia-differentiated neuronal stem cells (NSCs) isolated from mouse models of MSD and MPSIIIA. TFEB overexpression resulted in a striking reduction of alcian blue-stained GAGs in both MSD and MPSIIIA NSC-derived glial cells (Figure 5A). The latter result was further confirmed by pulse-and-chase experiments using H3-glucosamine to label GAGs, showing a significant reduction of the levels of labeled GAGs after 48 hr of chase in both MSD and MPSIIIA NSC-derived glial cells overexpressing TFEB (Figure 5B). Finally, EM analysis revealed that TFEB-mediated clearance of GAGs in TFEB-overexpressing MSD and MPS-IIIA cells was associated with both significant reduction of cellular vacuolization and recovery of normal cellular morphology (Figure 5C).
Figure 5.
TFEB Overexpression Reduces Storage in LSD Cells
(A) NSCs isolated from the cerebral cortex of MSD and MPSIIIA mice were nucleofected with either a TFEB plasmid or with an empty plasmid and cultured on coverslips. Alcian blue was then used to stain the GAGs.
(B) Wild-type, MSD, and MPSIIIA NSCs were nucleofected either with a TFEB plasmid or with an empty plasmid. After 16 hr, cells were pulsed with H3-glucosamine in differentiation medium and chased for the indicated time-points. Cell extracts obtained at different time points were quantified to determine the levels of labeled GAGs.
(C) Glia-differentiated MSD and MPS-IIIA NSCs were nucleofected with either TFEB or with an empty vector, fixed on glutaraldehyde, and processed for standard electron microscopy. The number of vacuoles per cell was counted from 20 different cells and displayed as mean ± SEM.
(D) TFEB promotes clearance of lipofuscin in fibroblasts from a patient with Batten disease. Cells were transfected with a vector carrying TFEB-Ruby (continuous red staining). After 24 hr, cells were examined by live imaging confocal analysis. Cells with increased TFEB (i.e., cells with red signal in the picture and outlined by dashed white lines in the middle panel) display highly reduced levels of lipofuscin (punctate green signal) and a normal cellular morphology compared with nontransfected cells (i.e., cells with intense green staining).
(E) Human Pompe disease fibroblasts were transfected with either TFEB or with an empty vector, loaded with the fluorescent sugar 2-NBDG, and analyzed by epifluorescence microscopy.
(F) Secretion efficiency of radioactive GAGs was measured in the culture medium of NSCs from MSD mice. Cells were nucleofected with either TFEB or an empty vector after pulse-chase incorporation of H3-glucosamine. Scale bars represent 100 μm (A), 10 μm (C, MSD cells), 500 nm (C, MPSIIIA cells), and 10 μm (D). Data represent mean ± SEM; ∗p < 0.05 (B, C, and F).
The involvement of Ca2+ in TFEB-mediated reduction of pathologic storage was confirmed by the inhibition of GAG clearance after the addition of BAPTA to TFEB overexpressing NSCs (Figure S4C). Consistent with this observation, viral-mediated overexpression of MCOLN1 in MSD glia-differentiated NSCs reduced GAG accumulation, but to a lesser extent compared with TFEB (Figure S4D), suggesting that Ca2+ elevation is required but not sufficient to mediate TFEB effects on lysosomal exocytosis.
We extended these studies to other types of LSDs associated with the storage of different types of lysosomal substrates. Neuronal ceroid lipofuscinoses (NCL) and glycogenosis are characterized by the accumulation of lipofuscin and glycogen, respectively (Beratis et al., 1978; Persaud-Sawin et al., 2007; Saftig, 2003). We found that TFEB overexpression strongly reduced lipofuscin autofluorescence in cells from a murine model of a juvenile form of NCL, known as Batten disease, due to mutations of the CLN3 gene (Figure 5D). Similarly, the accumulation of glycogen labeled with the exogenous fluorescent sugar 2-NBDG (Louzao et al., 2008) was significantly reduced upon TFEB overexpression in fibroblasts from a patient affected by glycogenosis type II, Pompe disease (Figure 5E). However, TFEB overexpression in mucolipidosis IV due to mutations in the MCOLN1 gene failed to induce cellular clearance, consistent with the involvement of MCOLN1 in TFEB-mediated induction of lysosomal exocytosis and clearance (Figure S4E). These data indicate that induction of lysosomal exocytosis promotes cellular clearance in diseases due to accumulation of different types of lysosomal substrates. Notably, all four diseases tested (i.e., MSD, MPS-IIIA, Batten, and NCL) are due to deficiency of proteins whose activity is involved in crucial steps of different catabolic pathways. In addition, the cells analyzed were derived from patients and murine models carrying either null mutations or mutations that severely inactivate protein function. Therefore, it is unlikely that clearance of lysosomal substrates in these cells is due to either the enhancement of the activity of the defective enzymes, or to an overall induction of lysosomal catabolic processes, again suggesting that clearance is mediated by exocytosis. This was confirmed by the observation of an increase in GAG secretion, measured by H3-GAG, in cell culture medium from MSD cells upon TFEB overexpression (Figure 5F).
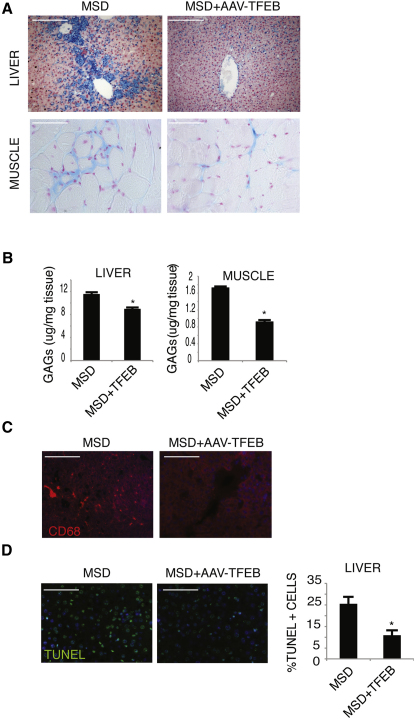
TFEB Delivery Rescues Tissue Pathology in a Mouse Model of MSD
We tested whether in vivo overexpression of TFEB in a mouse model of MSD, a severe type of LSD, had similar effects on cellular clearance. To this end, we injected systemically an adeno-associated type 2/9 virus (AAV2/9) that carries TFEB-3xflag into adult MSD mice. One month after injection, several tissues were collected to monitor transduction efficiency and GAG storage. AAV-mediated TFEB delivery resulted in efficient TFEB transduction (Figure S5) and significant reduction of GAG staining in liver and skeletal muscles, as detected by alcian blue staining (Figure 6A) and GAG quantification (Figure 6B). Subsequently, we investigated whether TFEB-mediated clearance of GAGs resulted in the reduction of the pathologic hallmarks of MSD, such as macrophage infiltration and apoptosis (Settembre et al., 2007). We found a striking reduction of CD68-positive cells in AAV-TFEB injected MSD mice compared with untreated mice (Figure 6C). Most importantly, we also observed a significant reduction of TUNEL-positive cells (Figure 6D). These results indicate that TFEB activation of lysosomal exocytosis reduced both primary accumulation of GAGs and secondary pathological processes associated with LSDs such as inflammation and cell death.
Figure 6.
TFEB Overexpression Reduces Storage and Tissue Pathology in a Mouse Model of MSD
(A) Alcian blue staining of GAG content in skeletal muscle and liver from mice injected systemically with either an AAV2/9-CMV-GFP or with an AAV2/9-CMV-TFEB3xflag viral vector.
(B) Quantitative analysis of GAG content in skeletal muscle and liver of MSD mice injected with either an AAV2/9-CMV-GFP or with an AAV2/9-CMV-TFEB3xflag viral vector. GAG content was displayed as mg of GAGs/mg of tissue extract. At least four mice per group were analyzed for each tissue examined (∗p < 0.05). Data are mean ± SEM.
(C) TFEB reduces inflammation in the liver of MSD mice. Macrophages and macrophage-related inflammatory cells were detected in liver sections from mice injected systemically with either an AAV2/9-CMV-GFP or with an AAV2/9-CMV-TFEB3xflag viral vector by immunofluorescence analysis using an antibody against CD68.
(D) Reduction of TUNEL-positive cells (arrows) in 4-month-old MSD mice injected with an AAV2/9-CMV-TFEB3xflag viral vector compared with age-matched MSD noninjected mice. At least four mice per group were analyzed for each tissue examined). Data are mean ± SEM; ∗p < 0.05 (B and D). Scale bars represent 100 μm (A, C, and D).
Conclusions
This study demonstrates that lysosomal exocytosis is transcriptionally regulated by TFEB, a master gene for lysosomal biogenesis and autophagy (Sardiello et al., 2009; Settembre et al., 2011). TFEB modulates lysosomal exocytosis by triggering intracellular Ca2+ elevation through the cation-channel MCOLN1. Cells may use this mechanism to coordinate lysosomal catabolic and secretory functions in response to specific stimuli. Our data also indicate that lysosomal exocytosis can be exploited to promote cellular clearance in lysosomal storage diseases, suggesting an alternative strategy to treat disorders due to intracellular storage, such as LSDs and common neurodegenerative diseases. Several approaches, such as gene delivery, pharmacological induction of TFEB, and target gene activation, could be exploited to promote cellular clearance in target tissues. These strategies will have to be tested by long-term in vivo studies in animal models to verify the therapeutic potential of this discovery.
Experimental Procedures
Cell Culture, Transfection, and VSV Infection
HeLa, Cos7 cells, human fibroblasts, and mouse embryonic fibroblasts from mouse models of MLIV (S7), MPSIIIA (S7), and MSD were transfected using PolyFect Transfection Reagent (QIAGEN, Hilden, Germany) or lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's protocols. TFEB-3xFLAG HeLa stable cell lines (CF7) was previously described (Sardiello et al., 2009). The shMCOLN1 HeLa cell line was generated by infection with MCOLN1 lentiviral shRNA. Neuronal progenitor cells were isolated from cortices of WT and MSD and MPSIIIA pups (P0) by using standard protocols (see Supplemental Experimental Procedures for details). NSCs were transfected by using nucleofection (Amaxa, Lonza, Walkersville, MD). Infection of control and CF7 HeLa cells with tsO45 strain of VSV was executed as described (Polishchuk et al., 2003).
Tannic Acid Treatment
To prevent fusion of exocytic organelles with PM, control and CF7 HeLa cells were incubated for 1 hr at 37°C in 20 mM HEPES-buffered DMEM supplemented with 0.5% tannic acid (TA). At the end of the incubation, TA-containing medium was removed and cells were fixed and processed for immuno-EM (see below). For immunofluorescense (IF) experiments VSV-infected cells were exposed subsequently to 40°C and 20°C temperature blocks to accumulate VSVG first in the ER and then in the Golgi. Then cells were shifted to 37°C to activate VSVG transport from the Golgi either in the presence or in the absence of 0.5% TA. One hour after the temperature shift to 37°C, the cells were fixed and processed for (IF) labeling (see below).
Surface LAMP1 Analysis
Cells (collected in phosphate-buffered saline [PBS] or grown on coverslips) were incubated with anti-rat LAMP1-1DB4 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at 4°C for 30 min. Cells were washed in PBS and fixed in 2% paraformaldehyde (PFA). Anti-LAMP1-1D4B–treated cells were further incubated with Alexa-594 conjugated anti–rat secondary antibodies (Molecular Probes, Invitrogen) for 30 min at room temperature. Finally, cells were analyzed on a confocal microscope (LSM510; Carl Zeiss, Inc., Thornwood, NY) equipped with Plan-Neofluar 63x immersion objective or on a FACs Aria Flow Cytometer (Becton Dickinson & Co., Mountain View, CA).
Immunofluorescence and Confocal Imaging
Transfected cells were grown on glass coverslips fixed with 4% paraformaldehyde (PFA, Sigma, St. Louis, MO), quenched with 50 mM NH4Cl, and permeabilized in blocking buffer (0.05% saponin/0.2% BSA in PBS/Ca/Mg). Coverslips were then incubated with appropriate primary antibodies described in Supplemental Experimental Procedures.
Tissues were fixed with 4% PFA and then subjected to a sucrose gradient (from 10 to 30%), before OCT embedding. Immunofluorescence analyses were performed on 10-μm-thick serial cryosections using appropriate primary antibodies (see Supplemental Experimental Procedures for details). The secondary antibodies were purchased from Molecular Probes (Invitrogen).
Images were taken on Vectashield mounted coverslips or sections using a confocal microscope (LSM510; Carl Zeiss, Inc.) equipped with a Plan-Neofluar 63x immersion objective (Carl Zeiss, Inc.).
Immunoblot and PM Isolation
PM proteins were isolated using the Cell Surface Protein Isolation kit (Pierce, Thermo Fischer Scientific Inc, Rockford, IL). Immunoblots were performed using standard protocols and primary antibodies as described in Supplemental Experimental Procedures. Proteins were quantified by the Bradford method.
Electron Microscopy and Immuno-Gold Analysis
Control and TFEB-overexpressing cells (both intact and TA-treated) were washed in PBS, and fixed in 0.05% glutaraldehyde dissolved in 0.2 M HEPES buffer (pH 7.4) for 30 min at room temperature. The cells were then postfixed for 2 hr in OsO4. After dehydration in graded series of ethanol, the cells were embedded in Epon 812 (FLUKA) and polymerized at 60°C for 72 hr. Thin sections were cut at the Leica EM UC6 and counterstained with uranyl acetate and lead citrate.
For immuno-gold HeLa and CF cells (both intact and TA-treated) were fixed with a mixture of 4% paraformaldehyde and 0.05% glutaraldehyde, labeled with a monoclonal antibody against LAMP1 according the gold-enhance protocol, embedded in Epon-812, and cut as described previously (Polishchuk et al., 2003).
EM images were acquired from thin sections using a Philips Tecnai-12 electron microscope equipped with an ULTRA VIEW CCD digital camera (Philips, Eindhoven, The Netherlands).
Quantification of vacuolization was performed using the AnalySIS software (Soft Imaging Systems GmbH, Muenster, Germany).
Evaluation of lysosome distance from the PM was done in EM images using the iTEM software (Soft Imaging Systems GmbH).
Selection of cells for quantification was based on their suitability for stereologic analysis, i.e., only cells sectioned through their central region (detected on the basis of the presence of Golgi membranes) were analyzed.
BSA-Gold
Electron microscopy and BSA immunogold were performed on HeLa control cells and overexpressing TFEB (CF7) according to the protocol described previously (Puri, 2009). See Supplemental Experimental Procedures for details.
Calcium Measurements by Confocal Imaging
TFEB-GFP-transfected cells were treated with FuraRed and analyzed according to the protocol described previously (Luciani et al., 2010).
Measurements of Ca2+ Concentration by Ratiometric Assays
HeLa cells were infected with either control adenovirus (Ad. Null) or adenovirus expressing TFEB (Ad. TFEB-FLAG). After 24 hr, cells were loaded with 20 μM Fura-2AM (Invitrogen) for 1 hr at 37°C. After washing, pseudocolor ratiometric images were acquired. The acquisition and analysis of the images were done using MetaFluor software (Molecular Devices, Sunnyvale, CA). Fura-2AM ratios (F340/F380) from cells infected with Ad. Null (n = 2584; where n represent the number of cells) or Ad. TFEB-FLAG (n = 2238) were used to calculate the cytoplasmic calcium concentration by using Fura-2AM calibration curves (see Supplemental Experimental Procedures for details).
Flow Cytometric Calcium Flux Assay
The assay was performed following the protocol described by Schepers et al. (2009) with some modifications as described in the Supplemental Experimental Procedures.
Enzymatic Activities
Enzyme activities were measured using the appropriate fluorimetric or colorimetric substrates as described in Supplemental Experimental Procedures.
Live Imaging
Live imaging was performed on WT and CF7 cells using a Carl Zeiss microscope (LSM 710) equipped with a perfusion, open and closed cultivation chamber fitted with a Tempcontrol and CO2-control device (Y module; Carl Zeiss). Acquisition was performed using ZEISS LSM 710 software. The trajectory and the mean velocity of vesicles were calculated by IMAGEJ using the LSM reader and particle tracking plug-ins. For details, see Supplemental Experimental Procedures.
GAG Analysis
GAGs were radiolabeled using H3-glucosamine hydrochloride (Perkin Elmer, 37.75 Ci/mmol, Boston, MA). Quantitative GAG analysis was performed according protocols described in (de Jong et al., 1989).
Alcian Blue Staining
Sections of paraffin-embedded tissues were stained with 1% Alcian blue (Sigma-Aldrich) and counterstained with Nuclear-Fast red (Sigma-Aldrich). See Supplemental Experimental Procedures for details.
In Situ Detection of Apoptotic Cells
TUNEL was performed by using the ApopTag Peroxidase In Situ Apoptosis Detection kit (Oncor, Gaithersburg, MD).
Acknowledgments
We thank P. Barba, J. Cancino, P. Colella, F. Donaudy, L. Pisapia, and A. Luciani for technical assistance. We thank E. Neufeld, B. Davidson, N. Andrews, C. Tam, and P. De Camilli for helpful discussions. We would like to acknowledge Elena Polishchuk and Anastasia Egorova for help in execution of microscopy experiments as well as Telethon Electron Microscopy Core Facility (IBP, CNR, Naples) and Integrated Microscopy Facility (IGB, CNR, Naples) for EM support. We also thank B. Lelouvier and the NHLBI Light Microscopy Core Facility for their help with the calcium experiments. We also thank G. Diez-Roux, G. Parenti, A. Luini, and A. De Matteis for comments on the manuscript. We acknowledge the support of the Italian Telethon Foundation (D.L.M., A.F., V.B., F.A., G.M., C.S., A.P., and A.B); the European Research Council Advanced Investigator grant number # 250154 (A.B); the European Commission under the FP7 EUCLYD project (Grant No. HEALTH-2007-A-201678); the Beyond Batten Disease Foundation (M.S and A.B); MPS Society (D.L.M, A.F.and A.B.). J.M. and R.P. are supported by the Intramural Research Program of the NIH, National Heart, Lung, and Blood Institute (NHLBI). In addition, we would like to thank the TIGEM AAV vector core for virus production.
Published online: September 1, 2011
Footnotes
Supplemental Information includes five figures, two movies, and Supplemental Experimental Procedures and can be found with this article online at doi:10.1016/j.devcel.2011.07.016.
Supplemental Information
Lysosomal movement was followed by live imaging in control HeLa-PCDNA3 cells transfected with a vector encoding the lysosomal membrane protein LAMP1 fused to GFP.
The movement of lysosomes was followed by live imaging in a stable HeLa clone expressing TFEB (HeLa-CF7 cells), and transfected with a vector encoding the lysosomal membrane protein LAMP1 fused to GFP.
References
- Andrews N.W. Regulated secretion of conventional lysosomes. Trends Cell Biol. 2000;10:316–321. doi: 10.1016/s0962-8924(00)01794-3. [DOI] [PubMed] [Google Scholar]
- Andrews N.W. Membrane repair and immunological danger. EMBO Rep. 2005;6:826–830. doi: 10.1038/sj.embor.7400505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabio A., Gieselmann V. Lysosomal disorders: from storage to cellular damage. Biochim. Biophys. Acta. 2009;1793:684–696. doi: 10.1016/j.bbamcr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Bargal R., Avidan N., Ben-Asher E., Olender Z., Zeigler M., Frumkin A., Raas-Rothschild A., Glusman G., Lancet D., Bach G. Identification of the gene causing mucolipidosis type IV. Nat. Genet. 2000;26:118–123. doi: 10.1038/79095. [DOI] [PubMed] [Google Scholar]
- Bargal R., Goebel H.H., Latta E., Bach G. Mucolipidosis IV: novel mutation and diverse ultrastructural spectrum in the skin. Neuropediatrics. 2002;33:199–202. doi: 10.1055/s-2002-34496. [DOI] [PubMed] [Google Scholar]
- Bassi M.T., Manzoni M., Monti E., Pizzo M.T., Ballabio A., Borsani G. Cloning of the gene encoding a novel integral membrane protein, mucolipidin-and identification of the two major founder mutations causing mucolipidosis type IV. Am. J. Hum. Genet. 2000;67:1110–1120. doi: 10.1016/s0002-9297(07)62941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beratis N.G., LaBadie G.U., Hirschhorn K. Characterization of the molecular defect in infantile and adult acid alpha-glucosidase deficiency fibroblasts. J. Clin. Invest. 1978;62:1264–1274. doi: 10.1172/JCI109247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blott E.J., Griffiths G.M. Secretory lysosomes. Nat. Rev. Mol. Cell Biol. 2002;3:122–131. doi: 10.1038/nrm732. [DOI] [PubMed] [Google Scholar]
- Bossi G., Griffiths G.M. CTL secretory lysosomes: biogenesis and secretion of a harmful organelle. Semin. Immunol. 2005;17:87–94. doi: 10.1016/j.smim.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Christensen K.A., Myers J.T., Swanson J.A. pH-dependent regulation of lysosomal calcium in macrophages. J. Cell Sci. 2002;115:599–607. doi: 10.1242/jcs.115.3.599. [DOI] [PubMed] [Google Scholar]
- de Jong J.G., Wevers R.A., Laarakkers C., Poorthuis B.J. Dimethylmethylene blue-based spectrophotometry of glycosaminoglycans in untreated urine: a rapid screening procedure for mucopolysaccharidoses. Clin. Chem. 1989;35:1472–1477. [PubMed] [Google Scholar]
- Dehay B., Bové J., Rodríguez-Muela N., Perier C., Recasens A., Boya P., Vila M. Pathogenic lysosomal depletion in Parkinson's disease. J. Neurosci. 2010;30:12535–12544. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K. Lysosomal diseases. In: Graham D.I., Lantos P.L., editors. Greenfield's Neuropathology. Arnold Hodder Publication; London: 2002. pp. 653–735. [Google Scholar]
- Dong X.P., Wang X., Shen D., Chen S., Liu M., Wang Y., Mills E., Cheng X., Delling M., Xu H. Activating mutations of the TRPML1 channel revealed by proline-scanning mutagenesis. J. Biol. Chem. 2009;284:32040–32052. doi: 10.1074/jbc.M109.037184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraldi A., Annunziata F., Lombardi A., Kaiser H.J., Medina D.L., Spampanato C., Fedele A.O., Polishchuk R., Sorrentino N.C., Simons K., Ballabio A. Lysosomal fusion and SNARE function are impaired by cholesterol accumulation in lysosomal storage disorders. EMBO J. 2010;29:3607–3620. doi: 10.1038/emboj.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Futerman A.H., van Meer G. The cell biology of lysosomal storage disorders. Nat. Rev. Mol. Cell Biol. 2004;5:554–565. doi: 10.1038/nrm1423. [DOI] [PubMed] [Google Scholar]
- Jaiswal J.K., Andrews N.W., Simon S.M. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J. Cell Biol. 2002;159:625–635. doi: 10.1083/jcb.200208154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao J.P., Li G., Auston D.A. Practical aspects of measuring intracellular calcium signals with fluorescent indicators. Methods Cell Biol. 2010;99:113–152. doi: 10.1016/B978-0-12-374841-6.00005-0. [DOI] [PubMed] [Google Scholar]
- Klein D., Büssow H., Fewou S.N., Gieselmann V. Exocytosis of storage material in a lysosomal disorder. Biochem. Biophys. Res. Commun. 2005;327:663–667. doi: 10.1016/j.bbrc.2004.12.054. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Mellman I. The biogenesis of lysosomes. Annu. Rev. Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- LaPlante J.M., Sun M., Falardeau J., Dai D., Brown E.M., Slaugenhaupt S.A., Vassilev P.M. Lysosomal exocytosis is impaired in mucolipidosis type IV. Mol. Genet. Metab. 2006;89:339–348. doi: 10.1016/j.ymgme.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Louzao M.C., Espiña B., Vieytes M.R., Vega F.V., Rubiolo J.A., Baba O., Terashima T., Botana L.M. “Fluorescent glycogen” formation with sensibility for in vivo and in vitro detection. Glycoconj. J. 2008;25:503–510. doi: 10.1007/s10719-007-9075-7. [DOI] [PubMed] [Google Scholar]
- Luciani A., Villella V.R., Esposito S., Brunetti-Pierri N., Medina D., Settembre C., Gavina M., Pulze L., Giardino I., Pettoello-Mantovani M. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat. Cell Biol. 2010;12:863–875. doi: 10.1038/ncb2090. [DOI] [PubMed] [Google Scholar]
- Matlin K.S., Simons K. Sorting of an apical plasma membrane glycoprotein occurs before it reaches the cell surface in cultured epithelial cells. J. Cell Biol. 1984;99:2131–2139. doi: 10.1083/jcb.99.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld E.F. Lessons from genetic disorders of lysosomes. Harvey Lect. 1979-1980;75:41–60. [PubMed] [Google Scholar]
- Newman T.M., Tian M., Gomperts B.D. Ultrastructural characterization of tannic acid-arrested degranulation of permeabilized guinea pig eosinophils stimulated with GTP-gamma-S. Eur. J. Cell Biol. 1996;70:209–220. [PubMed] [Google Scholar]
- Palmieri M., Impey S., Kang H., di Ronza A., Pelz C., Sardiello M., Ballabio A. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum. Mol. Genet. 2011 doi: 10.1093/hmg/ddr306. in press. Published online July 13, 2011. [DOI] [PubMed] [Google Scholar]
- Parton R.G., Prydz K., Bomsel M., Simons K., Griffiths G. Meeting of the apical and basolateral endocytic pathways of the Madin-Darby canine kidney cell in late endosomes. J. Cell Biol. 1989;109:3259–3272. doi: 10.1083/jcb.109.6.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud-Sawin D.A., Mousallem T., Wang C., Zucker A., Kominami E., Boustany R.M. Neuronal ceroid lipofuscinosis: a common pathway? Pediatr. Res. 2007;61:146–152. doi: 10.1203/pdr.0b013e31802d8a4a. [DOI] [PubMed] [Google Scholar]
- Platt F.M., Walkley S.U. Oxford University Press; New York: 2004. Lysosomal Disorders of the Brain. [Google Scholar]
- Polishchuk E.V., Di Pentima A., Luini A., Polishchuk R.S. Mechanism of constitutive export from the golgi: bulk flow via the formation, protrusion, and en bloc cleavage of large trans-golgi network tubular domains. Mol. Biol. Cell. 2003;14:4470–4485. doi: 10.1091/mbc.E03-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polishchuk R., Di Pentima A., Lippincott-Schwartz J. Delivery of raft-associated, GPI-anchored proteins to the apical surface of polarized MDCK cells by a transcytotic pathway. Nat. Cell Biol. 2004;6:297–307. doi: 10.1038/ncb1109. [DOI] [PubMed] [Google Scholar]
- Puri C. Loss of myosin VI no insert isoform (NoI) induces a defect in clathrin-mediated endocytosis and leads to caveolar endocytosis of transferrin receptor. J. Biol. Chem. 2009;284:34998–35014. doi: 10.1074/jbc.M109.012328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A., Caler E.V., Andrews N.W. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- Rodríguez A., Martinez I., Chung A., Berlot C.H., Andrews N.W. cAMP regulates Ca2+-dependent exocytosis of lysosomes and lysosome-mediated cell invasion by trypanosomes. J. Biol. Chem. 1999;274:16754–16759. doi: 10.1074/jbc.274.24.16754. [DOI] [PubMed] [Google Scholar]
- Rodríguez A., Webster P., Ortego J., Andrews N.W. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J. Cell Biol. 1997;137:93–104. doi: 10.1083/jcb.137.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saftig P. Springer; New York: 2003. Medical Intelligence Unit: Lysosomes. [Google Scholar]
- Sardiello M., Palmieri M., di Ronza A., Medina D.L., Valenza M., Gennarino V.A., Di Malta C., Donaudy F., Embrione V., Polishchuk R.S. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- Schepers E., Glorieux G., Dhondt A., Leybaert L., Vanholder R. Flow cytometric calcium flux assay: evaluation of cytoplasmic calcium kinetics in whole blood leukocytes. J. Immunol. Methods. 2009;348:74–82. doi: 10.1016/j.jim.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Settembre C., Annunziata I., Spampanato C., Zarcone D., Cobellis G., Nusco E., Zito E., Tacchetti C., Cosma M.P., Ballabio A. Systemic inflammation and neurodegeneration in a mouse model of multiple sulfatase deficiency. Proc. Natl. Acad. Sci. USA. 2007;104:4506–4511. doi: 10.1073/pnas.0700382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C., Fraldi A., Jahreiss L., Spampanato C., Venturi C., Medina D., de Pablo R., Tacchetti C., Rubinsztein D.C., Ballabio A. A block of autophagy in lysosomal storage disorders. Hum. Mol. Genet. 2008;17:119–129. doi: 10.1093/hmg/ddm289. [DOI] [PubMed] [Google Scholar]
- Settembre C., Di Malta C., Polito V.A., Garcia Arencibia M., Vetrini F., Erdin S., Erdin S.U., Huynh T., Medina D., Colella P. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker W.C., Weber T., Chapman E.R. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science. 2004;304:435–438. doi: 10.1126/science.1097196. [DOI] [PubMed] [Google Scholar]
- Vergarajauregui S., Puertollano R. Two di-leucine motifs regulate trafficking of mucolipin-1 to lysosomes. Traffic. 2006;7:337–353. doi: 10.1111/j.1600-0854.2006.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogalingam G., Bonten E.J., van de Vlekkert D., Hu H., Moshiach S., Connell S.A., d'Azzo A. Neuraminidase 1 is a negative regulator of lysosomal exocytosis. Dev. Cell. 2008;15:74–86. doi: 10.1016/j.devcel.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lysosomal movement was followed by live imaging in control HeLa-PCDNA3 cells transfected with a vector encoding the lysosomal membrane protein LAMP1 fused to GFP.
The movement of lysosomes was followed by live imaging in a stable HeLa clone expressing TFEB (HeLa-CF7 cells), and transfected with a vector encoding the lysosomal membrane protein LAMP1 fused to GFP.