Abstract
Background
Mantle cell lymphoma (MCL) is a rare variety of non-Hodgkin's lymphoma which originates from CD5+ B-cell population in the mantle zones of lymphoid follicles. Coexistence of such tumours in the axillary lymph nodes with invasive breast cancers without prior history of adjuvant chemotherapy or radiotherapy has not been previously reported in literature.
Case report
We report a rare case of breast cancer co-existing with stage I mantle cell lymphoma of the ipsilateral axillary lymph node detected fortuitously by population screening.
Conclusion
Though some studies have tried to prove breast carcinomas and lymphomas to share a common molecular or viral link, more research needs to be done to establish whether such a link truly exists.
Keywords: Lymphoma, breast, axilla, cancer, mantle cell, B-cell, carcinoma, neoplasm, lymph node, non Hodgkin's lymphoma
Background
Mantle cell lymphoma (MCL) is a rare type of B-cell lymphoma, which comprises 5–10% of all malignant lymphomas [1]. These tumours present in older adults with a median age of 65 and are often at an advanced stage (III or IV) when diagnosed [1,2]. Breast cancer patients have increased risks of Non-Hodgkin's lymphoma following treatment with radio and chemotherapy but coexistence of the two diseases is rare [3]. In the literature, no case of breast cancer occurring simultaneously with axillary mantle cell lymphoma has been reported previously and we present such a case here where both pathologies coexisted without any history of adjuvant therapy.
Report
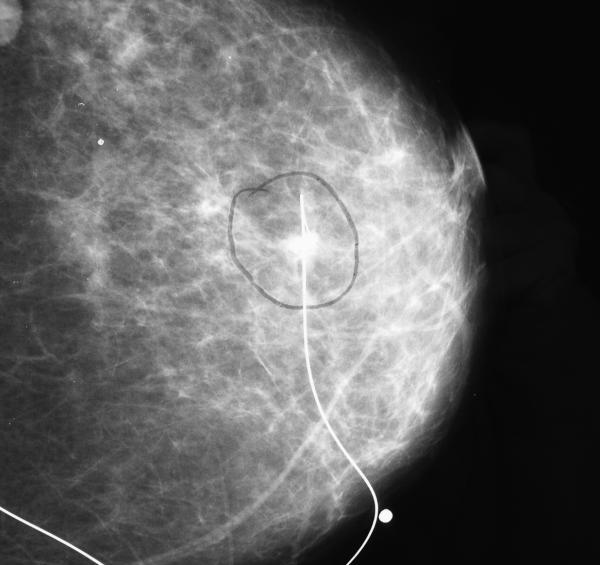
A 67-year-old postmenopausal lady presented herself for breast screening and the mammogram revealed a radiodense opacity over the upper outer quadrant of her left breast suspicious of carcinoma.
She had no previous breast problems and no family history of note. She was para 4, had her first pregnancy aged 22, had breast fed all her children, had been on oral contraceptive pills for 20 years and was taking Hormone Replacement Therapy (HRT) for 10 years which had been withdrawn a few months back. She was a non-smoker, a moderate drinker and had no significant medical problems. There was no palpable lump over her left breast and clinically she was aymptomatic without the presence of any B symptoms (night sweats, fever or loss of weight) and did not have lymphadenopathy or hepatosplenomegaly.
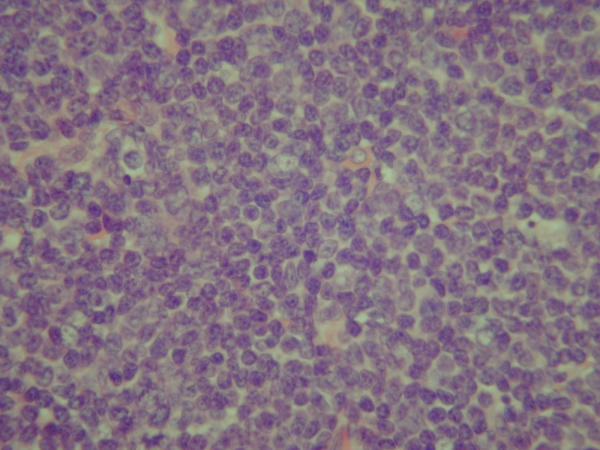
A core biopsy was undertaken which revealed grade II invasive ductal carcinoma of no special type. Her full blood count and liver function tests were within normal limits and chest X-ray was unremarkable. She underwent stereotactic needle localisation and wide local excision of the lump with axillary sampling. Per-operatively it was noted that two axillary lymph nodes were enlarged though soft in consistency. Histology of the breast lump revealed a 10 mm, grade 2 invasive ductal carcinoma that was positive for oestrogen receptors. The histology of the four axillary lymph nodes removed during the procedure showed diffuse proliferation of small to medium sized atypical lymphocytes with irregular nuclear contours, small nucleoli and scant cytoplasm suggesting malignant lymphoma. They were positive for pan B-cell antigens CD19, CD20, CD22 and negative for cytokeratin, oestrogen or progesterone receptors on immunohistochemistry and demonstrated Cyclin D1 (bcl 1) nuclear expression that was pathognomonic of mantle cell lymphoma.
She was referred to the haematology and oncology teams and subsequently underwent a computerised tomographic (CT) scan of her thorax, abdomen and pelvis, which revealed a small node in each axilla. A bone marrow aspiration was done which revealed no evidence of lymphoma. Repeat full blood count and biochemistry including Lactate Dehydrogenase (LDH) were also within normal limits. The histopathogy slides were reviewed and the initial diagnosis of mantle cell lymphoma was confirmed. She was subsequently started on Tamoxifen and adjuvant radiotherapy for definitive treatment of her breast cancer and following a multi-disciplinary meeting, a wait and watch policy with close monitoring by the surgical and oncology teams was employed with regard to her lymphoma given the fact that it was diagnosed at such an early stage.
Discussion
MCL is a rare type of B-cell lymphoma comprising 5–10% of all malignant lymphomas [1]. Because this lymphoma has been noticed and described recently, knowledge regarding MCL is limited. It was originally classified as a low-grade lymphoma, but recent reports of outcome indicate that most cases behave as intermediate-grade because it has neither the long survival of low-grade tumours nor the response to chemotherapy of high-grade lymphomas [2]. These tumours present in older adults (median age 65) and are commoner in men (male: female; 2.3:1) [2]. The disease is generally widespread at diagnosis(Stage III or IV) with invasion of the lymph nodes, spleen, bone marrow, gastrointestinal tract, peripheral blood and Waldeyer's ring and presence of B symptoms like weight loss, fever and night sweats [2].
A chromosomal translocation t(11; 14) involves the bcl-1 locus (chromosome 11) and the immunoglobulin heavy chain locus (chromosome 14) in such patients. The translocation causes overexpression of the proline-rich attachment domain PRAD-1 gene that encodes Cyclin D1, a promoter of progression of the cell cycle. The translocation can only be detected in half the patients, but the overproduction of Cyclin D1 is a diagnostic feature [4].
Four histologic subtypes have been described – Nodular, Diffuse, Mantle Zone and Blastic. It has been reported that the Mantle Zone variant may have a more indolent course than the other subtypes while Blastic appears to have the poorest prognosis [1,5]. Histologically, there is a mixture of small to medium sized atypical lymphocytes with slightly irregular contours, condensed chromatin, inconspicuous nucleoli and scanty cytoplasm [5]. They express a variety of B-cell antigens like CD19, CD20 and CD22 as well as CD5 and CD43 when stained for immunohistochemistry [2] and Cyclin D1 immunopositivity, if present, is pathognomonic [2,5].
MCL is currently incurable [6]. Treatment is mainly with chemotherapy though controversy rages whether CHOP (cyclophosphamide, adriamycin, vincristine, prednisone) or CVP (cyclophosphamide, vincristine, prednisone) regime is better. A small subgroup can be managed by observation only [1,6]. Two studies have shown survival benefits with an anthracyclin based regimen [6]. Overall, MCL responds to chemotherapy initially but usually recurs within 18 months and has the shortest median survival of all lymphomas with a median survival of 3–5 years regardless of treatment [5]. High-dose chemotherapy with stem cell transplant and allogenic bone marrow transplants has shown no survival benefits and research is currently under way to find a better treatment option [6].
Conclusion
Breast cancer patients have an increased risk of developing second malignancies following adjuvant chemotherapy and radiotherapy, including leukaemia and non-Hodgkin's lymphoma [3]. These generally appear 2–5 years following treatment. Co-existing mantle cell lymphoma and breast cancer has never been reported previously in literature and whereas this case might be a mere co-incidence, it also raises the possibility of a common link between these two cancers. Recent research has implicated the oncogenic Epstein-Barr virus as the possible agent responsible for spread of both invasive breast cancer and lymphomas through a latent antigen EBNA-3C which interacts specifically with the human metastatic suppressor protein Nm23-H1 and reverses its ability to stop migration of lymphoma and breast carcinoma cells [7]. Though more research needs to be done to come up with a definitive answer, it would be interesting to see if indeed there is a molecular link between such co-existing cancers triggering off neoplastic changes in genetically susceptible individuals.
Competing interests
None declared.
Author's contributions
SDR conceived the study, prepared the manuscript and conducted the literature search on this topic.
JAS prepared the histology and immunohistochemistry slides and reviewed them to confirm the diagnosis.
JS reviewed the radiology slides and performed the needle localisation on this patient before surgery.
SNS performed the initial operation, has been following up the patient in his clinic and participated in the final design and review of the manuscript
Figure 1.
Cranio-caudal view of mammogram showing needle localisation of the lump over upper outer quadrant of the left breast before wide local excision.
Figure 2.
Photomicrograph of the axillary lymphnode showing sheets of abnormal, irregular and pleomorphic lymphocytes with scant cytoplasm effacing normal lymphoid architecture (Haematoxylin and Eosin × 400).
Contributor Information
Subhajit Dutta Roy, Email: subhodr@yahoo.co.uk.
Joanna A Stafford, Email: Joanna.Stafford@mcht.nhs.uk.
John Scally, Email: John.Scally@mcht.nhs.uk.
S N Selvachandran, Email: selva@ntlworld.com.
References
- Argatoff L, Connors J, Klasa R, Horsman D, Gascoyne R. Mantle cell lymphoma: a clinicopathologic study of 80 cases. Blood. 1997;89:2067–2078. [PubMed] [Google Scholar]
- Fisher R. Mantle cell lymphoma: classification and therapeutic implications. Ann Oncol. 1996;7:S35–S39. doi: 10.1093/annonc/7.suppl_6.s35. [DOI] [PubMed] [Google Scholar]
- Harvey EB, Brinton LA. Second cancer following cancer of the breast. Natl Cancer Inst Monogr. 1985;68:99–112. [PubMed] [Google Scholar]
- Majlis A, Pugh W, Rodriguez M, Benedict W, Cabanillas F. Mantle cell lymphoma: correlation of clinical outcome and biologic features with three histologic variants. J Clin Oncol. 1997;15:1664–1671. doi: 10.1200/JCO.1997.15.4.1664. [DOI] [PubMed] [Google Scholar]
- Meusers P, Hense J, Brittinger G. Mantle cell lymphoma: diagnostic criteria, clinical aspects and therapeutic problems. Leukemia. 1997;11:S60–S64. [PubMed] [Google Scholar]
- Densmore JJ, Williams ME. Mantle cell lymphoma. Curr Treat Options Oncol. 2003;4:281–287. doi: 10.1007/s11864-003-0003-1. [DOI] [PubMed] [Google Scholar]
- Subramaniam C, Cotter MA, Robertson ES. Epstein-Barr virus nuclear protein EBNA-3C interacts with the human metastatic suppressor Nm 23-H1: A molecular link to cancer metastasis. Nature Medicine. 2001;7:350–355. doi: 10.1038/85499. [DOI] [PubMed] [Google Scholar]