Abstract
Objective This study examines relationships between affect and sleep in youth with affective disorders using ecological momentary assessment (EMA). Methods Participants included 94 youth, ages 8–16 (M = 11.73, 53% female) years with an anxiety disorder only (n = 23), primary major depressive disorder (with and without a secondary anxiety diagnoses; n = 42), and healthy controls (n = 29). A cell phone EMA protocol assessed affect and actigraphy measured sleep. Results The patterns of bidirectional relationships between affect and sleep differed across diagnostic groups. Higher daytime positive affect and positive to negative affect ratios were associated with more time in bed during the subsequent night for youth with primary depression and less time in bed for youth with anxiety only. More time asleep was associated with more positive affect for both diagnostic groups the following day. Conclusions This relationship may be important to consider in the treatment of youth affective disorders.
Keywords: adolescents, anxiety, children, depression, sleep
There is growing evidence to support a bidirectional relationship between sleep and affect in youth. This relationship can create a negative synergy of cyclical sleep and emotional disturbances where impairments in one area persistently maintain impairments in the other area (Carskadon & Acebo, 2002; Dahl, 1996; Dahl & Harvey, 2007; Dahl & Lewin, 2002; Fuligni & Hardway, 2006; Haynes et al., 2006). Both depressed mood and anxiety are associated with the development of sleep disturbances in youth (Forbes et al., 2008; Liu et al., 2008). In turn, sleep disturbances, such as symptoms of insomnia predict difficulties in affect regulation and psychopathology, such as anxiety and depression in youth (Alfano, Ginsburg, & Kingery, 2007; Gregory, Rijsdijk, Lau, Dahl, & Eley, 2009).
Reports of inconsistencies regarding specific aspects of sleep, self-report versus objective assessments, and the link between affective disorders have also emerged. Despite subjective reports of poor sleep in youth diagnosed with Major Depressive Disorder, objective sleep assessed with EEG showed adequate sleep (Bertocci et al., 2005; Forbes et al., 2008). In addition, objective and subjective measures of sleep were more similar to sleep reports in healthy youth, than youth diagnosed with anxiety disorders (Bertocci et al., 2005; Forbes et al., 2008). Youth with anxiety disorders exhibited less slow wave sleep and took longer to fall asleep than the healthy youth on objective sleep assessments. On subjective measures, long sleep latency was also reported; however, there were discrepancies between objective and subjective sleep problems, suggesting that youth with anxiety disorders might underestimate or underreport their sleep difficulties (Forbes et al., 2008).
Brent et al. (2008) found that sleep difficulties and fatigue were common symptoms among those with a partial but incomplete response to treatment for an affective disorder. Determining the extent to which sleep and affect interact will provide additional insight into target areas that may help cognitive behavioral treatments for affective functioning by reducing relapse rates. For instance, treatments that can relieve the added pressure of sleep disturbance on emotional systems for youth who already have difficulty activating and maintaining positive affect (Forbes & Dahl, 2005) may create more opportunities for behavioral and emotional improvements.
The goal of this study was to better understand directionality of these influences by examining the daily association of affect and sleep in youth with and without a current diagnosis of an affective disorder. The temporal sequencing in this unique approach allowed us to look at the distinct contribution of affect and sleep within and across affective disorders by examining how daytime affect impacts sleep the following night, as well as how nighttime sleep impacts the next day’s affect. Although there have been reports on sleep in youth with affective disorders from a large longitudinal study (Bertocci et al., 2005; Forbes et al., 2006, 2008; Gregory et al., 2010; Liu et al., 2008; Whalen et al., 2008); this study, however, stands alone based on the uniqueness of the research question, daily variation in the bidirectional relationship of sleep and affect in youth with and without affective disorder, the use of an innovative and ecologically valid affect and sleep methodology, and an advanced statistical approach.
We examined how reports of positive (PA) and negative (NA) affect assessed throughout the day were related to sleep. We also examined the PA/NA ratio. Increasing evidence points to the significance of this ratio as a more precise indicator of emotional health (Fredrickson & Losada, 2005). This rationale is based on the notion that PA may need to “outweigh” NA in order to overcome dysregulated affect and improve general mood. Therefore, mental health is associated with the extent to which one can elicit more positive than negative feelings and sustain this pattern over time. Conceptual models posit that youth with affective disorders, particularly youth with depression may have trouble activating positive affect and diminishing negative affect (Forbes & Dahl, 2005); therefore, the PA/NA ratio may be a more informative measure in which to assess the sleep and mood relationship within this population.
We hypothesized that, across all diagnostic groups (a) more NA and less PA during the day would be associated with worse sleep the following night (such as taking a long time to fall asleep and spending less time sleeping) and that (b) poor sleep would be associated with more NA and less PA the following day across all participants. As mentioned, research has shown that sleep and affect have a relationship; however, less is known about how this relationship may vary across diagnostic categories. For example, it may be that the relationship between affect and sleep is stronger among youth with affective disorders than among healthy youth, or vice versa. Because existing literature on this question is not available to guide-specific hypotheses, we conducted exploratory analyses examining whether the relationship between sleep and affect differed for youth diagnosed with major depressive disorder, anxiety disorder, and healthy controls.
Methods
Participants
This report includes data of a longitudinal clinical assessment study of neurobehavioral factors in pediatric affective disorder (Birmaher et al., 2000). The data presented are from the first 2 weeks of an 8-week protocol. Participants included 94 youth (53% female), ages 8–16 years (M = 11.73). The majority of the participants identified themselves as Caucasian (88%), with the remaining participants identified as African-American (7%), Hispanic (3%), and biracial (1%). Participants were divided into groups based on their current primary psychiatric diagnoses: (a) anxiety (ANX) n = 23 (Separation, GAD, & Social Phobia); (b) primary major depressive disorder (Primary MDD) n = 42, which was composed of youth with MDD only (n = 13) or MDD with secondary, comorbid anxiety disorders (n = 29; Separation, GAD, & Social Phobia); and (c) healthy controls n = 29.
Inclusion Criteria
Youth with an affective disorder met diagnostic criteria according to the American Psychiatric Association DSM-IV classification (2000). Healthy control youth were required to be free of any lifetime psychopathology and to have no first-degree relatives with a lifetime episode of any mood or psychotic disorder.
Exclusion Criteria
Since the youth in this study were originally recruited to participate in a larger study with a broad set of neurobehavioral assessments (Birmaher et al., 2000), the following exclusionary criteria applied at the time of enrollment: (a) use of any medication with central nervous system effects within the past 2 weeks or any lifetime use of fluoxetine, (b) significant medical illness, (c) extreme obesity (weight greater than 150% of ideal body weight) that would not allow the participants to fit comfortably in a magnetic resonance imaging (MRI) scanner, (d) IQ of 70 or less, (e) inordinate fear of intravenous needles (because of the need to draw blood for biological assays), and (f) specific learning disabilities. Subjects were also excluded if they had schizophrenic, schizoaffective, and bipolar disorders.
Procedure
The study was approved by the university’s Institutional Review Board. Participants were recruited from three sources: (a) community advertisements, (b) inpatient and outpatient clinics at a major medical center, and (c) referrals from other research studies or current participants. Youth and their parents were required to sign assents and informed consents, respectively. Structured diagnostic interviews were administered to establish lifetime and current youth psychiatric diagnoses and familial history of affective disorder. There was a 97% retention rate of subjects across the first 2 weeks of the study. Three participants dropped out of the larger study for reasons unrelated to these analyses after the baseline week and were excluded from these analyses. Qualifying participants for the larger study were invited to participate in a multifaceted protocol that included: (a) for participants with any psychiatric diagnosis, an 8-week open treatment protocol using CBT and/or SSRIs, based on the preference of the primary caregiver; (b) for all participants, a visit to the neurobehavioral laboratory during the baseline week of the study (Forbes et al., 2006; Ladouceur et al., 2005); and (c) also for all participants, a home assessment protocol that included ecological momentary assessment (EMA) and measures of sleep in the home environment collected in biweekly intervals over the 8-week study. The focus of this report is on sleep and mood data collected through the home assessment protocol at study entry.
Instruments
Structured Diagnostic Interviews
Each youth and his or her parent(s) were interviewed to determine the youth’s psychiatric history using the Schedule for Affective Disorders and Schizophrenia in School-Age Children—Present and Lifetime version (K-SADS-PL, Kaufman, Birmaher, Brent, & Rao, 1997). Other adult first-degree and second-degree relatives were assessed indirectly using a modified version of the Family History Interview (Weissman, Greenwald, Niño-Murcia, & Dement, 1997), with the youth’s parent(s) and other available relatives serving as informant(s). All interviews were carried out by trained BA- and MA-level research clinicians. Inter-rater reliabilities for diagnoses assessed during the course of this study were estimated to be k ≥ 0.70. The results of the interview were presented at a consensus case conference with a child psychiatrist, who reviewed the findings and preliminary diagnosis and provided a final diagnosis based on DSM-IV criteria.
Ecological Momentary Assessment
As part of the larger study, all participants completed a cell-phone EMA protocol designed to provide real-time data on behavior, emotion, and social context in the natural environment (Silk et al., 2011). Participants were given answer-only cellular phones on which they received calls from trained research assistants for 4-day intervals (Friday–Monday) over eight consecutive weeks (Axelson et al., 2003). These analyses include the first and second 4-day intervals over the first 2 weeks of the 8-week period. Participants were called 12 times between 4 p.m. Friday and 10 p.m. Monday, for a total of 24 calls. Due to scheduling and daytime activity constraints related to school, EMA, and sleep assessments were collected during periods when participants were not in school, which included after school hours and school nights (Sundays and Mondays) and weekend nights (Fridays and Saturdays). The present report focuses on the affect ratings from the calls. At each call, participants were asked to rate their current affect (How would you rate how happy you are right now?) on a 5-point scale (1—Not at all to 5—Extremely) from items selected from the Positive and Negative Affect Schedule for Children (PANAS-C; Watson, Clark & Tellegen, 1988). The four items selected for positive affect were (happy, joyful, excited, and energetic) and the four items selected for negative affect were (sad, angry, nervous, and upset). Global NA and PA scores were created by averaging across the negative and positive subscales for each day, and a daily ratio of global PA/NA was also computed from these means (Fredrickson & Losada, 2005).
Actigraphy
Actigraphs are wristwatch-like devices, worn 24 hr/day that provide an estimate of the sleep/wake cycle via movement. In order to directly correspond to the EMA, participants wore an Octagonal Basic Motionlogger wrist actigraph from Ambulatory Monitoring, Inc., on their non-dominant wrist during each of the data collection intervals, from Friday afternoon at 4 p.m. until they awoke Tuesday morning, removing it only for contact sports, swimming, and/or bathing. Actigraphs recorded continuously. Participants were instructed to press a button on the watch to indicate when they were in bed trying to go to sleep and when they woke up, which inserted a marker into the actigraph record. Actigraphy data were pre-processed and scored in 60-s epochs using ActionW2.5. Data was then downloaded and analyzed by trained coders that identified sleep onset and offset to generate sleep parameters. Data were processed using the Cole–Kripke procedure (Cole, Kripke, Gruen, Mullaney & Gillin, 1992). Sleep onset was the first minute of the first 20-min contiguous period of low activity scored as sleep. Sleep offset was the last minute of low activity scored as sleep during the night. Coders were trained by scoring records collectively, then by individually scoring the same records, comparing and discussing discrepancies. The correlations between actigraphy and EEG-defined sleep estimates range from 0.85 to 0.95 in youth (Sadeh, Raviv, & Gruber, 2000). We focused on five commonly used sleep variables to ascertain how youth were sleeping at night: (a) the total amount of time spent in bed at night (TIB); (b) total sleep time (TST); (c) sleep onset latency, or the time between trying to sleep and sleep onset, represented in minutes (SOL); (d) wakefulness after sleep onset, the total number of minutes spent awake at night after sleep onset (WASO); and (e) sleep efficiency (SEf), which is the percentage of time spent sleeping while in bed. Higher sleep efficiency represents spending more time in bed sleeping than not sleeping.
Data Analysis
Our goal was to conduct exploratory analyses examining the relationship between sleep and affect for youth diagnosed with major depressive disorder and youth diagnosed with an anxiety disorder compared to healthy controls. Preliminary analyses showed significant differences between the diagnostic groupings on gender (primary MDD: 63% female; ANX only: 35% female; control: 58% female), age (primary MDD: 12; ANX only: 10; control: 12), and SES (primary MDD: 37.0; ANX only: 40.0; control: 47.5) (all p < .05); therefore, these variables were centered and included as covariates in additional analyses. Demographics were not associated with sleep or affect. Prior to the analyses, an additional three participants were removed due to having limited data that could skew the results; this included one participant with only 1 day of actigraphy data across both time points, one participant with only two nights of actigraphy, one night per time point, and one participant with only two successful EMA calls across both time points. A total of 94 participants’ data were utilized for these analyses. A total of 8 days of data collection across 2 weeks were included in the analyses, the average number of days of data per participants was eight (Range 6–8), approximately 4 days/week (Friday through Monday). There were a total of 12 EMA calls conducted over 4 days across each week of the study. The participants had an average of 11 successful calls per each 4-day interval (range 7–12). There was an average of four nights of actigraphy data per participant during baseline and three nights during Week 1 (Range 2–4). Table I displays the means and standard deviation for the sleep and affect variables.
Table I.
Means and Standard Deviation for the Sleep and Affect Variables Across the Study
| Sleep |
Affect |
|||||||
|---|---|---|---|---|---|---|---|---|
| Sleep latency | Wake after sleep onset | Total sleep time | Time in bed | Sleep efficiency | Negative affect | Positive affect | PA/NA | |
| Low-risk controlsa | 16 m (14 m)**ab,*ac | 43 m (30 m)**ab | 8 h 32 m (1 h 11 m)*ab | 9 h 16 m (1 h 11 m) | 92% (6%)**ab | 1.07 (.16)**ab,*, ac | 3.05 (.74)**ab | 2.92 (.81)**ab |
| Primary MDDb | 22 m (22 m) | 57 m (42 m)*bc | 8 h 9 m (1 h 30 m) | 9 h 6 m (1 h 35 m) | 89% (8%)*bc | 1.31 (.40)**bc | 2.51 (.99)**bc | 2.09 (1.08)**bc |
| ANX onlyc | 21 m (20 m) | 48 m (33 m) | 8 h 23 m (1 h 13 m) | 9 h 11 m (1 m 13 m) | 91% (6%) | 1.14 (.25) | 3.20 (1.09) | 2.94 (1.14) |
Note. Standard deviations are adjusted for nonindependence of observation. Affect scale: 1, not at all; 2, a little; 3, moderately; 4, quite a bit; 5, extremely.
Asterisks followed by a letter combination indicates a statistically significant difference between the means (SD) of the two groups.
The diagnostic groups are each designated with a letter: aLow-risk controls, bPrimary MDD, and cANX only.
*p < .05, **p < .01.
We accounted for the nesting of assessments (i.e., calls nested within days within participants), by conducting repeated measures linear mixed effects models with study day as a random effect and study week as a repeated measure. To address hypothesis 1, poor daytime affect predicting more sleep difficulties the following night, we conducted a series of lagged linear mixed effects models in which the fixed effects included diagnostic group and affect (PA, NA, or the PA/NA ratio) (day to sleep) (Table II). To address hypothesis 2, more nighttime sleep difficulties predicting worse affect the following day, we conducted a similar series of lagged linear mixed effects models in which the fixed effects included diagnostic category and sleep (SOL, WASO, TST, TIB and SEf) (Table III). Across both series of models, individual affect variables were paired with individual sleep variables (i.e., NA to SOL or SOL to NA), depending on the predicted direction. We explored the relationship between sleep and affect for youth with and without affective disorders by examining the interaction between diagnostic group and affect predicting sleep from affect and affect from sleep. The repeated covariance type was specified as heterogeneous compound symmetry. The beta weights and standard errors are presented in hours and fractions of hours. All analyses were conducted with SPSS 18.
Table II.
Summary of Fixed Effects for Each Mixed Effects Models of Daytime Affect Predicting Nighttime Sleep
| SOL | WASO | TST | TIB | SEf | |
|---|---|---|---|---|---|
| 1. Negative affect | 0.218 | 0.439 | 0.112 | 0.290 | 0.070 |
| Diagnostic group | 1.97 | 5.13** | 0.835 | 0.049 | 2.05 |
| Diagnostic group × negative affect | 1.57 | 3.66* | 0.231 | 0.347 | 0.888 |
| 2. Positive affect | 0.160 | 0.036 | 0.232 | 0.326 | 0.011 |
| Diagnostic group | 1.83 | 0.760 | 4.90** | 5.01* | 0.216 |
| Diagnostic group × positive affect | 1.54 | 0.091 | 2.31 | 3.30* | 0.209 |
| 3. PA/NA ratio | 0.032 | 0.073 | 0.188 | 0.893 | 0.001 |
| Diagnostic group | 0.503 | 0.604 | 3.94* | 5.98** | 0.169 |
| Diagnostic group × PA/NA ratio | 0.270 | 1.21 | 1.30 | 3.65* | 1.05 |
Note. Variables: SOL, WASO, TST, TIB, SEf. Diagnostic Groups: MDD, ANX, and Controls. The df for diagnostic group and the interaction was 2, the df for the affect rating was 1. F-values presented for those components of the models are adjusted for age, gender, and SES.
*p < .05, **p < .01.
Table III.
Summary of Fixed Effects for Each Mixed Effects Models of Nighttime Sleep Predicting Daytime Affect
| Negative Affect | Positive Affect | PA/NA Ratio | |
|---|---|---|---|
| 1. SOL | 0.527 | 0.102 | 0.023 |
| Diagnostic group | 81.05** | 6.97** | 6.86** |
| Diagnostic group × SOL | 7.31* | 4.16* | 1.88 |
| 2. WASO | 2.08 | 0.539 | 0.022 |
| Diagnostic group | 23.22** | 19.60** | 10.41** |
| Diagnostic group × WASO | 6.19** | 0.061 | 0.835 |
| 3. TST | 0.449 | 0.327 | 0.152 |
| Diagnostic group | 4.74* | 7.73** | 7.06** |
| Diagnostic group × TST | 0.223 | 3.20* | 2.31 |
| 4. TIB | 0.011 | 0.027 | 0.207 |
| Diagnostic group | 5.23** | 7.56** | 6.81** |
| Diagnostic group × TIB | 0.603 | 2.51 | 1.94 |
| 5. SEf | 3.08† | 0.160 | 0.004 |
| Diagnostic group | 1.73 | 0.094 | 0.329 |
| Diagnostic group × SEf | 2.07 | 0.038 | 0.669 |
Note. Diagnostic Groups: MDD, ANX, and Controls. The df for diagnostic group and the interaction was 2, the df for each sleep variable was 1. F-values presented for those components of the models are adjusted for age, gender, and SES.
†p < .10; *p < .05; **p < .01.
Results
Daytime Affect predicting the following Nighttime Sleep
In the models predicting nighttime sleep from affect during the day, several main effects emerged. Reports of greater negative affect during the day were associated with less time spent awake that same night for youth diagnosed with primary MDD (B = –.68, SE = .34, p = .05); however, this was not the case for youth with ANX only or healthy controls. Reports of PA and the PA/NA ratio during the day were associated with the amount of time spent sleeping and the amount of time spent in bed the following night; however, the relationship differed across diagnostic group. For youth diagnosed with primary MDD, reports of more daytime PA was associated with less time in bed (B = −1.57, SE = .50, p = .003) and less total sleep time (B = –1.74, SE = .56, p = .003) and a higher PA/NA ratio during the day was associated with less time in bed (B = −1.45, SE = .42, p = .002) and less sleep time (B = –1.31, SE = .50, p = .010). These relationships were not observed for the youth with ANX only or healthy controls (Table II). Main effects for the models predicting sleep latency, time spent awake at night, and sleep efficiency from affect were not significant.
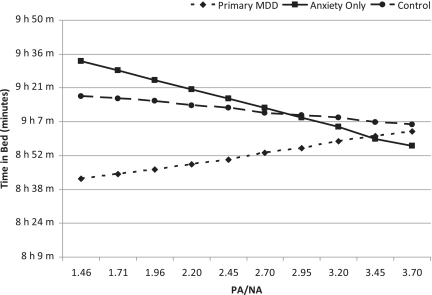
An interaction between daytime negative affect and the amount of time spent awake the following night with diagnostic group emerged. In order to interpret the role of the diagnoses in these interactions, post hoc linear mixed models were conducted with negative affect predicting the sleep variable for each diagnosis. The model revealed that as negative affect increased, the time spent awake the following night decreased for youth diagnosed with primary MDD (B = –.40, SE = .31, p = .02) and increased the following night for you diagnosed with ANX only (B = .33, SE = .12, p = .05). Interactions between PA and the PA/NA ratio and diagnostic category predicting time spent in bed the following night were also revealed. As positive affect increased during the day, the amount of time spent in bed the following night decreased for the youth with ANX only (B = –.28, SE = .12, p = .03) and increased for the youth with primary MDD (B = .43, SE = .17, p = .01). The same pattern was observed for PA/NA, increases in the PA/NA ratio during the day were associated with less time in bed the following night for the youth with ANX only (B = –.27, SE = .11, p = .03) and more time in bed the following night for the youth with primary MDD (B = .42, SE = .16, p = .01) (Figure 1). There were no interactions found for diagnostic category and affect predicting sleep latency or sleep efficiency.
Figure 1.
The ratio of PA/NA predicting the amount of time spent in bed that night. Plotted values are adjusted for the mean levels for age, gender, and socioeconomic status.
Nighttime Sleep Predicting Affect the Following Day
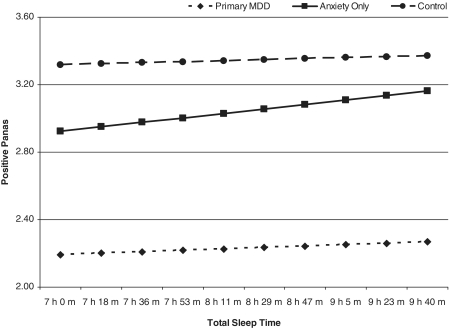
In the models predicting daytime affect from the previous night’s sleep, significant interactions emerged between diagnostic group and the amount of time to fall asleep, time awake at night, and total sleep time predicting affect the next day (Table III). In order to interpret the interactions, post hoc linear mixed models were conducted examining this relationship within each diagnostic group. A longer sleep latency was associated with less PA the following day (B = –.00018, SE = .000065, p = .006) and more NA the following day (B = 000056, SE = .000016, p = .001) for youth diagnosed with primary MDD. More time awake at night was related to more NA the following day for youth with ANX only (B = .000048, SE = .000162, p = .003). More time spent sleeping was associated with more PA the following day for youth with primary MDD (B = .000032, SE = 000013, p = .016) and youth with anxiety (B = .00003, SE = .000017, p = .050) (Figure 2). There were no main effects for nighttime sleep predicting affect the following day for any of the sleep variables. There was, however, a main effect for diagnostic category in the models predicting NA, PA, and the PA/NA ratio for all sleep models, except sleep efficiency; however, the effects of diagnosis on affect in daily life are the focus of a separate investigation (see Silk et al., 2011) and was not a focus of the current investigation.
Figure 2.
The amount of time spent sleeping at night predicting positive affect the following day. Plotted values are adjusted for the mean levels for age, gender, and socioeconomic status.
Discussion
These results demonstrate a bidirectional relationship between daytime affect and sleep for youth diagnosed with affective disorders but not for healthy control youth. However, the nature of the bidirectional relationship differs between youth diagnosed with anxiety only and youth diagnosed with primary depression. For daytime affect predicting sleep the following night, reports of more positive affect and increases in the PA/NA ratio during the day was associated with less time in bed the following night for youth with anxiety only. Whereas reports of more positive affect and increases in the PA/NA ratio during the day was associated with more time spent in bed the following night, and more daytime negative mood was associated with less time spent awake the following night for youth with primary depression. For nighttime sleep predicting affect the following day, less time awake at night was associated with less negative mood reports the following day for youth with anxiety only, whereas reduced sleep latency was associated with more positive reports of mood and less negative mood reports the following day for youth with primary depression. For both youth with anxiety and youth with primary depression, more total sleep time was associated with more positive reports of mood the next day. An association was not found for the healthy control youth.
Perhaps the differential response to affect during the day and sleep across diagnoses is associated with specific symptomology of affective disorders. Youth with anxiety only and youth with primary depression had an opposite relationship with positive daytime affect and time spent in bed the following night. Although time in bed is often thought of as an index of getting more sleep, it also includes time spent in bed at night not sleeping. It may be that when anxious youth have more positive affect during the day they spend less time in bed worrying and ruminating at bedtime that night. However, sleep may be impacted in a different manner for youth with primary depression, who may not display the same types of bedtime worry often associated with anxious youth. The bed may represent a safe place to “hide away” or withdraw as opposed to a place associated with worry and distress. Perhaps, the bed or the bedroom is an escape, a time and a place to feel safe and comfortable.
Sleep efficiency, a representation of time spent in bed sleeping versus being awake and total sleep time were not predicted by daytime affect reports for any diagnostic group. This lack of relationship may support the notion that daytime affect’s impact on nighttime sleep is related more to aspects surrounding sleep, such as the bed and bedroom. However, future research may want to evaluate this notion with EMA assessments around bedtime or with the use of subjective sleep assessments that are able to attain aspects of sleep not represented by actigraphy, such as sleep quality, which may provide further insights into these findings.
When examining nighttime sleep predicting reports of affect the following day, we found that better sleep, more time spent sleeping, less time getting to sleep, and less time awake at night was associated with better daytime affect the following day for youth with affective disorders. These findings are consistent with existing research suggesting that good sleep is associated with better mood (Dahl, 1996; O’Brien & Mindell, 2005). As mentioned, research has shown that youth with depression have difficulties sustaining positive affect (Forbes & Dahl, 2005). The predictive relationship between sleep and affect may help create the framework for an intervention designed to help youth with depression and other affective disorders toward the process of maintaining positive emotions.
The emergence of the affect and sleep relationship in clinical samples demonstrates that the impact of affect on sleep and vice versa is salient for youth diagnosed with an affective disorder; however, a relationship was not observed for healthy control youth. It is worth mentioning that the data show a similar pattern toward a relationship between worse sleep and worse daytime affect for the controls. There is clear evidence of developmental changes in sleep and sleep behaviors beginning at the onset of puberty, such as reduced sleep at night and a shift in the sleep/wake cycle towards later bedtimes (Carskadon & Acebo, 2002). Insufficient sleep, which is often a result of these sleep changes can have a negative impact on affect for all youth (Dahl & Lewin, 2002). The age of the sample may provide a possible explanation. The mean age was just less than 12 years, thus we may be capturing a time in development prior to these pubertal sleep changes. Accordingly, the healthy controls had better sleep compared to the youth with affective disorders, which could be related to age, puberty, healthier sleep habits, the lack of an affective disorder, or any combination of these factors. This may have contributed to the lack of significant effects. In addition, individuals differ in their response to sleep problems and feelings of positive and negative affect; however, the ability to keep affect and sleep schedules relatively stable across days may be an example of more appropriate affective regulation that is difficult in youth diagnosed with an affective disorder. Future studies would benefit from further examination of sleep patterns in youth with and without affective disorder. This would be particularly important with puberty, as this is typically a time of increased sleep and affective struggles.
Although many of the youth with primary depression also had a secondary anxiety disorder, youth with primary depression had worse sleep and consistently reported less positive affect and more negative affect than the youth with anxiety only. Current and forthcoming cognitive and behavioral treatments may benefit from directly addressing the relationship between sleep and affect. Intervention programs that target sleep in youth with depression and anxiety disorders, may promote improvements in emotion regulation in addition to improved sleep and less varied sleep schedules. Furthermore, it may be of interest to gauge how youth who are receiving treatment for depression or anxiety utilize their bed at night, as well as how bedtime and sleep are conceputalized by the youth. For instance, do these youth think of sleep as worrisome or think of their beds as a safe haven from the distress of depression? A greater understanding of impressions of sleep may help tease out some of the discrepancies found in objective and subjective reports of sleep for youth with anxiety and/or depression (Forbes, 2008). This type of information could also help to provide future treatment goals; for instance, would it be more beneficial to address sleep or affect at the start of treatment for youth with depression? Longitudinal studies could explore whether reports of affect improve in conjunction with improving aspects of sleep for youth with primary depression to determine if these reporting biases are malleable and responsive to changes in sleep.
Despite these findings, several limitations of the present study should be addressed. First, because the sample of youth diagnosed with primary depression without comorbid secondary anxiety was relatively small, we were unable to include analyses on participants with major depressive disorder only. Interestingly, the differences found between the youth with primary depression (who often had comorbid anxiety) and youth with pure anxiety disorders suggest that the symptoms related to the depressive disorder may be driving the affect/sleep relationship. In future studies it would be beneficial to include a larger sample to help tease out the sleep/affect relationship with co-morbid diagnoses. Second, we included a relatively broad age range and did not have adequate power to test interactions between diagnostic group, gender, and pubertal development. Although we controlled for age, we did not examine the role of puberty in this relationship due to the sample size and the number of analyses conducted. However, given the puberty-related changes in sleep and depression, future studies would benefit from investigating the role of puberty to better understand the developmental impact of the sleep and affect association. Third, in order to gather data congruent with EMA, actigraphy was only obtained during the same time period, yielding only four nights of data which included some weeknights (Sunday–Monday) and weekend nights (Friday–Saturday). Previous studies have shown that a minimum of seven days is ideal to obtain a view of sleep and sleep patterns (Acebo et al., 1999). Although we only had a few days of actigraphy, we did gather this information across 2 weeks, which provides an extended sampling of sleep across time in our sample. However, this limits our ability to generalize this relationship during the entire school week for youth with affective disorders. In addition, the EMA data was averaged across time points throughout the day. It may be beneficial to examine morning and bedtime affect ratings separately along with overall daily affect to better understand the sleep and affect relationship. Future studies would also benefit from longer continuous periods of subjective and objective sleep data collection across days and time as a means to understand and evaluate sleep and affect patterns. Fourth, due to the number of multiple models that were examined and the possibility of increased type 1 error, the results should be interpreted with some level of caution. Finally, future studies may benefit from utilizing more complex time-series models that would provide unique information about this relationship, such as affect fluctuation within a day, variability across time for different diagnostic groups, and different affect, sleep, and environmental variables.
This study also has several notable strengths that contribute to our understanding of sleep and affect in young people with affective disorders. This study utilized an innovative, methodological approach of an intensive EMA protocol that provides data on affect in the youth’s natural environment. Studies have shown that even adults are inaccurate at reporting retrospective affect (Wilson, Meyers, & Gilbert, 2001). For youth, it is difficult to accurately remember feelings and sleeping behaviors over the course of a week. Obtaining this information via EMA allows a more accurate representation of these variables and decreases memory bias (Stone, Shiffman, & DeVries, 1999). An additional benefit of the EMA assessments is that the daily calls to the participants reinforce adherence to other measures in the study, in this case actigraphy. This study advances previous work by focusing on a rigorously diagnosed clinical sample of youth with depression and anxiety and utilizing an approach that provides data collected in home environments over an extended period of time. Compared to subjective reports, the use of an objective measure of sleep helps to create a more accurate portrayal of sleep behavior across multiple time points from which sleep information was collected. As a result, the unique study design allowed for the detection of this bidirectional relationship, which holds both clinical and methodological implications to better understand the interaction between daily affect and sleep. In all, this study's findings suggest that sleep and daily affect are interrelated, both from day to night and from night to day, for youth diagnosed with major depressive and anxiety disorders.
Funding
This research was supported by National Institute of Mental Health (NIMH) (Grant PO1 MH41712; N.D.R., PI, R.E.D., Co-PI).
Conflicts of interest: None declared.
Acknowledgments
The methods of this study are based on a study first reported in (Rao et al., 2002). The authors are grateful to Laura Trubnick, Jennifer Jakubcak, and Michelle Bertocci, and the staff of the Child and Adolescent Neurobehavioral Laboratory for their invaluable role in assessing the participants in this study.
References
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Wolfson A R, Hafer A, Carskadon MA. Estimating sleep patterns with activity monitoring in children and adolescents: How many nights are necessary for reliable measures? Sleep. 1999;22(1):95–103. doi: 10.1093/sleep/22.1.95. [DOI] [PubMed] [Google Scholar]
- Alfano C, Ginsburg G, Kingery J. Sleep-related problems among children and adolescents with anxiety disorders. Journal of American Academy of Child & Adolescent Psychiatry. 2007;46(2):224. doi: 10.1097/01.chi.0000242233.06011.8e. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Third Edition Revised (DSM-III-R) Washington, DC: 1987. [Google Scholar]
- Axelson D, Bertocci M, Lewin D, Trubnick L, Birmaher B, Williamson D, Ryan N D, Dahl RE. Measuring mood and complex behavior in natural environments: Use of ecological momentary assessment in pediatric affective disorders. Journal of Child and Adolescent Psychopharmacology. 2003;13(3):253–266. doi: 10.1089/104454603322572589. [DOI] [PubMed] [Google Scholar]
- Bertocci M, Dahl R, Williamson D, Iosif A, Birmaher B, Axelson D, Ryan N D. Subjective sleep complaints in pediatric depression: A controlled study and comparison with EEG measures of sleep and waking. Journal of American Academy of Child & Adolescent Psychiatry. 2005;44(11):1158. doi: 10.1097/01.chi.0000179057.54419.17. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Dahl R, Williamson D, Perel J, Brent D, Axelson D, Kaufman J, Dorn L D, Stull S, Rao U, Ryan N D. Growth hormone secretion in children and adolescents at high risk for major depressive disorder. Archives of General Psychiatry. 2000;57(9):867–872. doi: 10.1001/archpsyc.57.9.867. [DOI] [PubMed] [Google Scholar]
- Brent D, Emslie G, Clarke G, Wagner KD, Asarnow J R, Keller M, Vitiello B, Ritz L, Iyengar S, Abebe K, Birmaher B, Ryan N, Kennard B, Hughes C, DeBar L, McCracken J, Strober M, Suddath R, Spirito A, Leonard H, Melhem N, Porta G, Onorato M, Zelazny J. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: The TORDIA randomized controlled trial. Journal of the American Medical Association. 2008;299(8):901–913. doi: 10.1001/jama.299.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon M A, Acebo C. Regulation of sleepiness in adolescents: Update, insights, and speculation. Sleep. 2002;25(6):606–614. doi: 10.1093/sleep/25.6.606. [DOI] [PubMed] [Google Scholar]
- Cole R J, Kripke D F, Gruen W, Mullaney D J, Gillin J C. Automatic sleep wake identification from wrist activity. Sleep. 1992;15:461–469. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- Dahl R. The regulation of sleep and arousal: Development and psychopathology. Development and Psychopathology. 1996;8:3–27. [Google Scholar]
- Dahl R, Harvey A. Sleep in children and adolescents with behavioral and emotional disorders. Sleep Medicine Clinics. 2007;2(3):501–511. [Google Scholar]
- Dahl R, Lewin D. Pathways to adolescent health sleep regulation and behavior. Journal of Adolescent Health. 2002;31(6):175–184. doi: 10.1016/s1054-139x(02)00506-2. [DOI] [PubMed] [Google Scholar]
- Forbes E, Bertocci M, Gregory A, Ryan N, Axelson D, Birmaher B, Dahl R E. Objective sleep in pediatric anxiety disorders and major depressive disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(2):148. doi: 10.1097/chi.0b013e31815cd9bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E E, Dahl R E. Neural systems of positive affect: Relevance to understanding child and adolescent depression? Development and Psychopathology. 2005;17:827–850. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E E, Silk J S, Thompson W K, Bertocci M A, Axelson D A, Birmaher B, Williamson D E, Ryan N D, Dahl R E. Major depressive disorder, pubertal maturation, and adolescents’ subjective positive affect in natural settings. 2011 Manuscript submitted for publication. [Google Scholar]
- Forbes E, Williamson D, Ryan N, Birmaher B, Axelson D, Dahl R. Peri-sleep-onset cortisol levels in children and adolescents with affective disorders. Biological Psychiatry. 2006;59(1):24–30. doi: 10.1016/j.biopsych.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson B L, Losada M F. Positive affect and the complex dynamics of human flourishing. American Psychologist. 2005;60:678–686. doi: 10.1037/0003-066X.60.7.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuligni A J, Hardway C. Daily variation in adolescents’ sleep, activities, and psychological well-being. Journal of Research on Adolescence. 2006;16(3):353–377. [Google Scholar]
- Gregory A, Cousins J C, Forbes E, Trubnick L, Ryan N D, Axelson D, Birmaher B, Sadeh A, Dahl R E. Sleep items in the child behavior checklist: A comparison with sleep diaries, actigraphy and polysomnography. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(5):499–507. doi: 10.1016/j.jaac.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory A, Rijsdijk F, Lau J, Dahl R, Eley T. The direction of longitudinal associations between sleep problems and depression symptoms: A study of twins aged 8 and 10 years. Sleep. 2009;32(2):189. doi: 10.1093/sleep/32.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes P L, Bootzin R R, Smith L, Cousins J, Cameron M, Stevens S. Sleep and aggression in substance-abusing adolescents: Results from an integrative behavioral sleep-treatment pilot program. Sleep. 2006;29(4):512–520. [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U. Schedule for Affective Disorders and Schizophrenia fo School-Age Children-(K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Ladouceur C, Dahl R, Williamson D, Birmaher B, Ryan N, Casey B. Altered emotional processing in pediatric anxiety, depression, and comorbid anxiety-depression. Journal of Abnormal Child Psychology. 2005;33(2):165–177. doi: 10.1007/s10802-005-1825-z. [DOI] [PubMed] [Google Scholar]
- Liu X, Forbes E, Ryan N, Rofey D, Hannon T, Dahl R. Rapid eye movement sleep in relation to overweight in children and adolescents. Archives of General Psychiatry. 2008;65(8):924. doi: 10.1001/archpsyc.65.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien E, Mindell J. Sleep and risk-taking behavior in adolescents. Behavioral Sleep Medicine. 2005;3(3):113–133. doi: 10.1207/s15402010bsm0303_1. [DOI] [PubMed] [Google Scholar]
- Rao U, Dahl R E, Ryan N D, Birmaher B, Williamson D E, Rao R, Kaufman J. Heterogeneity in EEG sleep findings in adolescent depression: Unipolar versus bipolar clinical course. Journal of Affective Disorders. 2002;70(3):273–280. doi: 10.1016/s0165-0327(01)00396-2. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Raviv A, Gruber R. Sleep patterns and sleep disruptions in school-age children. Developmental Psychology. 2000;36(3):291–300. doi: 10.1037//0012-1649.36.3.291. [DOI] [PubMed] [Google Scholar]
- Silk J S, Forbes E E, Whalen D J, Jakubcek J L, Thompson W K, Ryan N D, Axelson D A, Birmaher B, Dahl R E. Understanding daily emotional and social dynamics in depressed youth: A Cell-Phone Ecological Momentary Assessment Study. 2011 doi: 10.1016/j.jecp.2010.10.007. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruyt K, O'Brien L M, Cluydts R, Verleye G B, Ferri R. Odds, prevalence and predictors of sleep problems in school-aged normal children. Journal of Sleep Research. 2005;14:163–176. doi: 10.1111/j.1365-2869.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- Stone A, Shiffman S, DeVries M. Ecological momentary assessment. Well-being: The foundations of hedonic psychology. 1999:26–39. [Google Scholar]
- Weissman M, Greenwald S, Niño-Murcia G, Dement W. The morbidity of insomnia uncomplicated by psychiatric disorders. General Hospital Psychiatry. 1997;19(4):245–250. doi: 10.1016/s0163-8343(97)00056-x. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark L A, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Whalen D, Silk J, Semel M, Forbes E, Ryan N, Axelson D, Birmaher B, Dahl R E. Caffeine consumption, sleep, and affect in the natural environments of depressed youth and healthy controls. Journal of Pediatric Psychology. 2008;33(4):358–367. doi: 10.1093/jpepsy/jsmo86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T, Meyers J, Gilbert D. Lessons from the past: Do people learn from experience that emotional reactions are short-lived? Personality and Social Psychology Bulletin. 2001;27(12):1648. [Google Scholar]