Table 1.
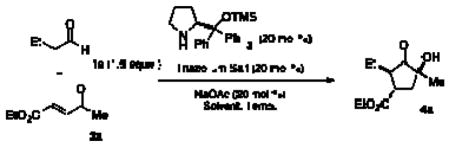 | ||||||
|---|---|---|---|---|---|---|
| Solvent | Triazolium salt | Temp. (°C) | Yield (%) | ee (%)b | drb | |
| 1c | MeOH |
 5 |
23 | 0 | -- | -- |
| 2c | CHCl3 | 5 | 23 | 29 | 96 | 3:1:<1:<1 |
| 3d | CHCl3 | 5 | 23 | 35 | 96 | 2:1:<1:<1 |
| 4d | CHCl3 | 5 | 60 | 89 | 96 | 5:1:<1:<1 |
| 5d | CHCl3 |
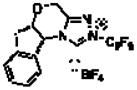 6 |
60 | 87 | 95 | 19:1:<1:<1 |
| 6d | CHCl3 |
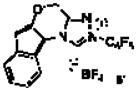 6′ |
60 | 59 | 93 | 4:1:<1:<1 |
See supporting information for general procedure.
Enantioselectivity and diastereoselectivity were determined by GC.
20 mol % Triazolium salt was added after full consumption of 2a.
20 mol % Triazolium salt was added at the beginning of the reaction.
