Abstract
Although the decision between stem cell self-renewal and differentiation has been linked to cell-cycle modifications, our understanding of cell-cycle regulation in stem cells is very limited. Here, we report that FBF/Pumilio, a conserved RNA-binding protein, promotes self-renewal of germline stem cells by repressing CKI-2Cip/Kip, a Cyclin E/Cdk2 inhibitor. We have previously shown that repression of CYE-1 (Cyclin E) by another RNA-binding protein, GLD-1/Quaking, promotes germ cell differentiation. Together, these findings suggest that a post-transcriptional regulatory circuit involving FBF and GLD-1 controls the self-renewal versus differentiation decision in the germline by promoting high CYE-1/CDK-2 activity in stem cells, and inhibiting CYE-1/CDK-2 activity in differentiating cells.
Keywords: Cip/Kip, CKI-2, FBF, MEX-3, Pumilio
Introduction
Several lines of evidence suggest that the decision to self-renew or differentiate is closely connected to the cell-cycle status. While pluripotent cells typically display rapid G1-to-S phase transitions in the cell cycle, differentiation correlates with slower divisions, longer gap phases and introduction of cell-cycle checkpoints (Orford and Scadden, 2008; Lange and Calegari, 2010). Also, factors promoting the G1-to-S phase transition impede differentiation (Kato and Sherr, 1993; Skapek et al, 1995; Richard-Parpaillon et al, 2004; Lange et al, 2009). Despite growing evidence for the importance of cell-cycle regulation in self-renewal and differentiation, the connection between the cell cycle and critical stem cell factors is yet to be understood.
A conserved feature of the animal germline is a key role of RNA-binding proteins in controlling the self-renewal versus meiosis/differentiation decision. For example, members of the PUF (Pumilio and FBF) family of RNA-binding proteins maintain germline stem cells (GSCs) in diverse organisms (Forbes and Lehmann, 1998; Crittenden et al, 2002; Salvetti et al, 2005). In C. elegans, two redundant PUF proteins, FBF-1 and -2 (collectively referred to as FBF), are required for stem cell maintenance, as GSCs are lost from fbf-1 fbf-2 mutant gonads (Crittenden et al, 2002). Previously, FBF was shown to repress expression of the differentiation-promoting protein GLD-1 (Suh et al, 2009). This finding provided a paradigm for compartmentalization of factors regulating self-renewal versus differentiation, but did not explain how these factors affect the cell cycle.
Recently, we have shown that the differentiation-promoting role of GLD-1 involves translational repression of cye-1 (Cyclin E) mRNA, which prevents ectopic activation of CYE-1/CDK-2 in germ cells undergoing meiosis and their reversal to self-renewal (Biedermann et al, 2009). Here, we show that repression of CKI-2, a member of the Cip/Kip family of cyclin-dependent kinase inhibitors (CKIs; Buck et al, 2009), is important for the maintenance of GSCs. We demonstrate that CKI-2 is repressed in GSCs and that this repression is mediated by conserved elements in the cki-2 mRNA 3′UTR that recruit FBF. Importantly, while GSCs are lost from fbf-1 fbf-2 gonads (Crittenden et al, 2002), GSCs are restored in fbf-1 fbf-2 gonads upon depletion of CKI-2, suggesting that FBF-mediated repression of CKI-2 is critical for maintenance of GSCs. To our knowledge, these findings establish the first direct link between a conserved stem cell factor and the cell cycle in adult stem cells. Because Cip/Kip CKIs in worms and other animals inhibit Cdk2 activity (Besson et al, 2008), we propose that FBF and GLD-1 regulate the self-renewal versus differentiation decision, at least in part, by patterning CYE-1/CDK-2 activity; ensuring high levels of CYE-1/CDK-2 in GSCs to promote self-renewal, and low levels in cells undergoing meiosis to promote differentiation.
Results
The Cip/Kip protein CKI-2 is repressed in GSCs
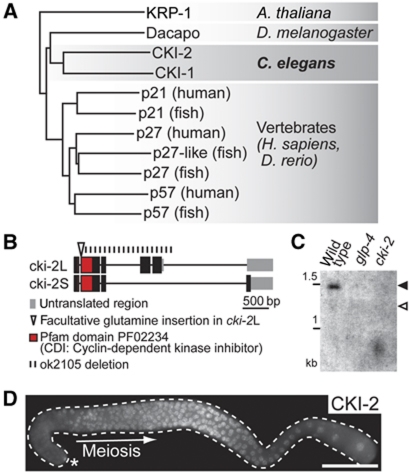
The C. elegans genome encodes two members of the Cip/Kip family: CKI-1 and CKI-2 (Figure 1A). While CKI-1 is required in somatic blast cells for the proper timing of cell-cycle withdrawal (Hong et al, 1998), CKI-2 is not essential, having a minor role during vulval development (Buck et al, 2009). By semiquantitative RT–PCR and immunofluorescent detection, we found that of the two Cip/Kip proteins, CKI-2 is the predominant CKI in the adult germline (Figure 1C and D; Supplementary Figure S1A and B). Immunodetection studies revealed that CKI-2 protein was absent from GSCs and became expressed in cells entering meiosis (Figure 1D). In both cki-2(ok2105) mutants and cki-2(RNAi) animals, CKI-2 protein is essentially absent (Figure 1B and C; unpublished observation). Yet, the animals are viable and fertile (Buck et al, 2009), suggesting that CKI-2 is not essential for the entry into and progression through meiosis. However, since the Cip/Kip proteins tend to be absent from pluripotent cells (Ramalho-Santos et al, 2002; Ginis et al, 2004), we wondered whether repression of CKI-2 in GSCs is important for their maintenance.
Figure 1.
CKI-2Cip/Kip is repressed in germline stem cells. (A) CKI-1 and CKI-2 are C. elegans Cip/Kip proteins. Phylogenetic tree of Cip/Kip proteins obtained with ClustalW (default settings). Protein sequences were retrieved from Uniprot. (B) cki-2 is alternatively spliced. By 3′RACE analysis, cki-2 encodes two alternatively spliced mRNA isoforms, cki-2 L and S (1316 and 951 nt long, encoding a 259 and 175 amino acid-long protein, respectively). The ok2105 deletion removes most of the cki-2 coding sequence. (C) cki-2L mRNA predominates and is germline specific. Northern blot of cki-2 mRNA isolated from young (non-gravid) wild-type, glp-4 and cki-2(ok2105) mutants using a probe detecting both isoforms. cki-2L (black arrowhead, 1316 nt plus poly-A tail) predominates, while cki-2S (empty arrowhead, 951 nt+poly-A tail) is hardly detectable. cki-2 mRNA is absent from germline-less glp-4(bn2ts) animals and from ok2105 mutants. (D) CKI-2 protein is absent from germline stem cells. Immunodetection of CKI-2 in a dissected gonad (outlined). CKI-2 is expressed upon meiotic entry but not in the distal-most stem cells, which are here and in the remaining figures marked by an asterisk. Scale bar: 50 μm.
CKI-2 repression is mediated through the 3′UTR of its mRNA
The self-renewal of C. elegans GSCs depends on GLP-1/Notch signalling, but its relevant targets remain unclear (Kimble and Crittenden, 2007). To determine if Notch signalling regulates CKI-2 expression, we measured cki-2 mRNA levels by quantitative RT–PCR in gonads in which GLP-1 was either active or inactive. Specifically, we dissected gonads from gld-1 gld-2; glp-1(gf) (gf: gain-of-function) animals (GLP-1 ON) and from gld-1 gld-2; glp-1(lf) (lf: loss-of-function) animals (GLP-1 OFF) (Priess et al, 1987; Kodoyianni et al, 1992; Kadyk and Kimble, 1998; Pepper et al, 2003; Supplementary Figure S1C). We found that cki-2 mRNA abundance increased moderately in the absence of GLP-1/Notch signalling (Supplementary Figure S1D). Surprisingly, CKI-2 protein was absent from both GLP-1 ON and GLP-1 OFF gonads (unpublished observation). Thus, though GLP-1 signalling affects cki-2 mRNA abundance (directly or indirectly), an additional regulatory mechanism preventing CKI-2 protein expression must exist.
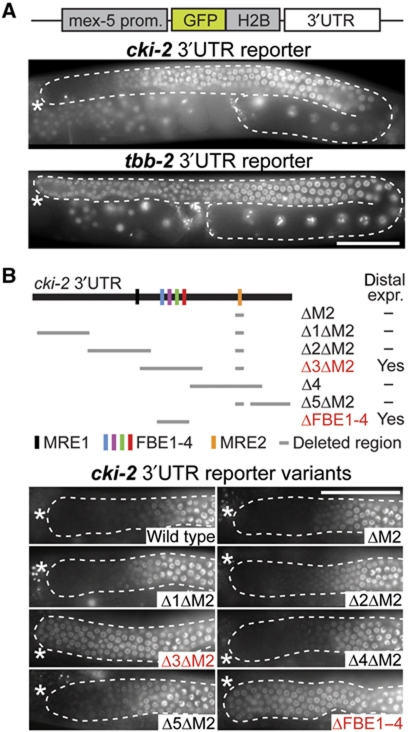
Post-transcriptional regulation is common in germ cells and is often mediated by the 3′UTR of mRNAs (Merritt et al, 2008). To test if the cki-2 3′UTR mediates repression in GSCs, we produced single-copy integrated transgenic lines expressing a GFP–H2B reporter from a constitutive (mex-5) germline promoter. When this reporter was fused to the housekeeping tbb-2 (tubulin) 3′UTR, it was expressed throughout the germline. In contrast, a reporter fused to the cki-2 3′UTR was repressed in stem cells (Figure 2A). Thus, a 3′UTR-based mechanism may be sufficient to repress CKI-2 in GSCs.
Figure 2.
A cluster of FBEs in the cki-2 3′UTR mediates repression in germline stem cells. (A) The cki-2 3′UTR mediates repression in stem cells. A GFP–H2B reporter (depicted schematically) is repressed in stem cells when coupled to the cki-2 3′UTR, but expressed ubiquitously when coupled to the non-regulated tbb-2 3′UTR. (B) A region required for repression harbours potential PUF binding elements. Graphical representation of the cki-2 3′UTR (top). Shown are relative locations of MRE1, MRE2 and FBE1–4 elements, the extent of deletions tested in reporter constructs, and the effect of the deletion on distal reporter expression. Deleting FBE1–4 is sufficient for distal derepression of the reporter (bottom). Partial (B) or complete (A) gonads (outlined) from live animals are shown. Scale bars: 50 μm.
Putative FBF-binding elements in cki-2 3′UTR mediate reporter repression in GSCs
To identify RNA elements regulating cki-2 mRNA expression, we aligned cki-2 3′UTRs from various nematode species. We observed several blocks of homology. One contains a putative MEX-3 recognition element (MRE; Pagano et al, 2009); an additional MRE was found in a non-conserved region. Another conserved region contains several potential FBF-binding elements (FBEs) that are recognized by PUF proteins (Wickens et al, 2002; Supplementary Figure S2). Because PUF and MEX-3 proteins have been implicated in germ cell proliferation (Crittenden et al, 2002; Ariz et al, 2009), we tested whether their putative binding sites were required for cki-2 repression.
We produced a set of reporter constructs fused to the cki-2 3′UTR containing overlapping deletions (Figure 2B); these reporters were each integrated as a single copy into a defined genomic locus using MosSCI (Frøkjaer-Jensen et al, 2008). Removing the conserved MEX-3 binding site (ΔM2) was not sufficient to cause reporter derepression in wild-type GSCs. Consistently, the wild-type 3′UTR reporter remained repressed in GSCs in the mex-3 mutant (unpublished observation). Nevertheless, since MEX-3 can promote proliferation in sensitized backgrounds (Supplementary Figure S3C and D; Ariz et al, 2009), we initially combined the MEX-3 binding site mutation with larger deletions (Δ1–5). We found that deleting fragments 3 and M2 caused reporter derepression (Figure 2B). Importantly, also a smaller deletion removing only the potential FBEs (ΔFBE1–4) but leaving MRE2 intact was equally sufficient to derepress the reporter (Figure 2B). Removing only the highly conserved motif FBE1 resulted in barely detectable derepression (unpublished observation), indicating that multiple FBEs within the cluster contribute to repression of cki-2 mRNA. Thus, though MRE2 is conserved, associates with MEX-3, and may contribute to cki-2 regulation under special circumstances (Supplementary Figure S3), it is FBEs that are normally critical for cki-2 mRNA repression in GSCs.
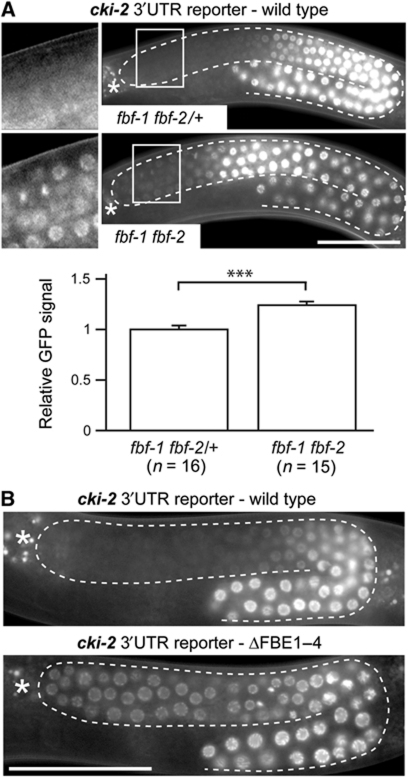
FBF represses the cki-2 3′UTR reporter in GSCs
To complement the above analysis, we tested if a reporter containing the wild-type cki-2 3′UTR was derepressed in fbf-1 fbf-2 gonads. Since FBF maintains self-renewal in the adult germline, adults have no GSCs in which to assay reporter repression. For this reason, and as has been done previously (Merritt and Seydoux, 2010), we assayed reporter expression in fbf-1 fbf-2 mutant L4 larvae, at which stage FBF is not yet essential. In contrast to wild-type animals that repressed the reporter in GSCs, we observed its partial derepression in fbf-1 fbf-2 animals (Figure 3A). To determine if the residual repression depends on the region containing putative FBEs, we analysed expression of the ΔFBE1–4 reporter at the same larval stage and found that it was fully derepressed (Figure 3B). Because another PUF protein, PUF-8, has been implicated in germ cell proliferation (Ariz et al, 2009), we tested whether PUF-8 contributes to cki-2 reporter repression. We found that RNAi-mediated depletion of PUF-8 neither derepressed the reporter (Supplementary Figure S4A), nor enhanced reporter derepression in fbf mutants (unpublished observation). These results demonstrate that FBEs mediate cki-2 repression in larval and adult GSCs, and suggest that an additional factor (apparently distinct from FBF, PUF-8 and MEX-3) contributes to repression of cki-2 in GSCs during larval development when FBF is not critical.
Figure 3.
FBF represses the cki-2 3′UTR reporter in stem cells. (A) The wild-type cki-2 3′UTR reporter is partially derepressed in dividing stem cells of fbf-1 fbf-2 mutant L4 animals. Gonads (outlined) from live fbf-1 fbf-2 mutant and fbf-1 fbf-2/+ heterozygote sibling L4 animals are shown. Boxed areas are magnified on the left. Quantification of GFP signal in distal-most cells is shown below (n=number of examined gonads, error bars=s.e.m.). ***Indicates the P-value of 1.298e-05 (see Materials and methods). (B) The FBE1–4 region mediates repression at the L4 stage. A cki-2 3′UTR reporter lacking the FBE1–4 region is derepressed in stem cells of L4 animals. Scale bars: 50 μm.
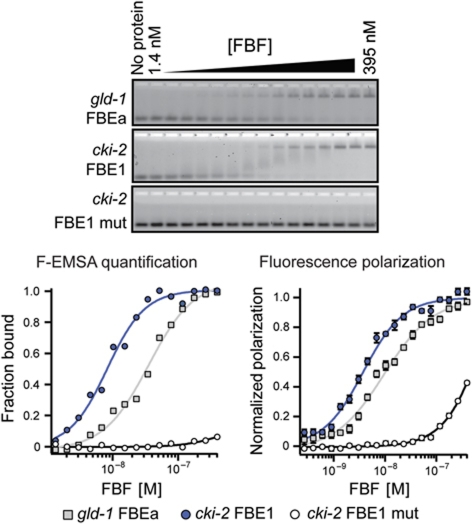
FBF binds the FBEs mediating cki-2 repression
To test if FBF associates with its putative binding elements in the cki-2 3′UTR, we used fluorescence electrophoretic mobility shift (F-EMSA) and fluorescence polarization (FP) assays (Pagano et al, 2009, 2011). Since FBF-1 and FBF-2 act redundantly (Lamont et al, 2004) and have similar RNA binding characteristics (Bernstein et al, 2005), these assays were performed only with FBF-2. Within the conserved region of the cki-2 3′UTR that mediates regulation of the reporter (Figure 2B), five UGU motifs are found in the context of four possible FBEs, FBE1 through 4 (Supplementary Figure S2). FBF-2 was found to bind with high affinity to FBE1; this binding is comparable to the previously described FBF affinity to FBEa, a binding element in the gld-1 3′UTR (Bernstein et al, 2005; Wang et al, 2009; Figure 4; Table 1). We also found that FBF-2 bound with modest affinity to FBE3 and FBE4 elements, and weakly to FBE2, which is the least conserved element (Supplementary Figure S2; Table 1). Mutating the UGU sequence to ACA eliminated FBF binding to these elements (Table 1). These experiments demonstrate that FBF directly binds FBEs that mediate repression of cki-2 in GSCs.
Figure 4.
FBF-2 associates with FBE1 in the cki-2 3′UTR. Top: Representative F-EMSA gels determining FBF-2 association with wild-type and mutated (mut) elements from the gld-1 and cki-2 3′UTRs. Bottom left: Quantification of gel shifts shown above. The fraction of bound RNA was plotted as a function of protein concentration and fitted to the Hill equation to determine the apparent equilibrium dissociation constant (Kd,app). Bottom right: Affinity as determined by fluorescence polarization assays. Polarization is plotted as a function of protein concentration and fitted to the Hill equation to determine Kd,app.
Table 1. Quantification of FBF-2 association with FBEa and FBE1–FBE4.
| Oligo name | Kd,app(GS) | Kd,app(FP) |
|---|---|---|
| gld-1 FBEa | 32±1 nM | 11±2 nM |
| cki-2 FBE1 | 26±12 nM | 5±1 nM |
| cki-2 FBE1 mut | > 500 nM | > 500 nM |
| cki-2 FBE2 | > 500 nM | > 500 nM |
| cki-2 FBE2 mut | > 500 nM | > 500 nM |
| cki-2 FBE3 | 240±8 nM | 114±2 nM |
| cki-2 FBE3 mut | NB | >500 nM |
| cki-2 FBE4 | 300±40 nM | 95±3 nM |
| cki-2 FBE4 mut | NB | >500 nM |
| Affinities of FBF-2 to indicated RNA elements as determined by F-EMSA and FP assays. NB, no binding. Kd,app, apparent equilibrium dissociation constant. For oligo sequences, see Supplementary Table S1. | ||
Removing CKI-2 rescues the stem cell loss observed in fbf-1 fbf-2 mutant gonads
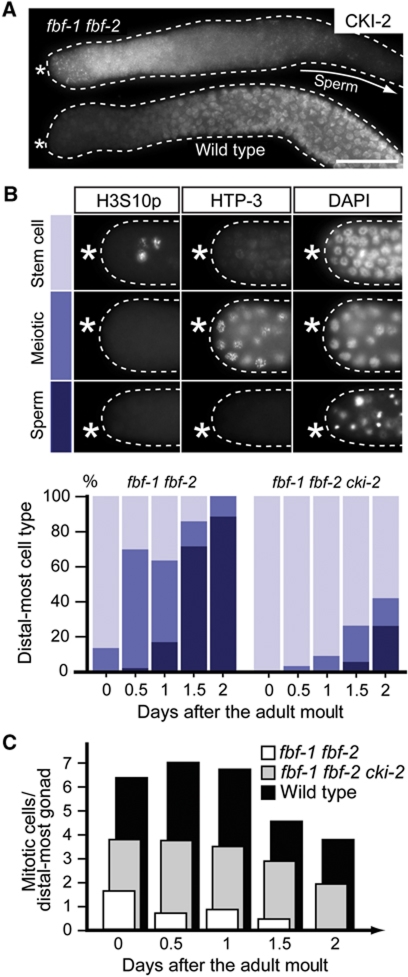
Expectedly, we found that CKI-2 was derepressed in the distal-most cells of fbf-1 fbf-2 mutant gonads (Figure 5A). To test if this ectopic expression of CKI-2 in fbf-1 fbf-2 gonads is responsible for the stem cell loss observed in these animals, we constructed fbf-1 fbf-1 cki-2 triple mutants. As previously observed, fbf-1 fbf-2 mutant animals rapidly lost GSCs after the adult moult (Crittenden et al, 2002; Figure 5B and C). In contrast, fbf-1 fbf-2 cki-2 adult animals retained GSCs beyond day 2 of adulthood (Figure 5B and C). This interaction appeared to be specific to FBF and CKI-2, as the cki-2 mutation did not suppress the reduced proliferation phenotype seen in puf-8(RNAi) (Supplementary Figure S4B) or puf-8(RNAi); mex-3 animals (Ariz et al, 2009; unpublished observation). Together, these results demonstrate that CKI-2 is repressed in GSCs by FBF, which binds a conserved regulatory region in cki-2 mRNA 3′UTR, and suggest that FBF-mediated repression of CKI-2 is critical for the maintenance of GSCs.
Figure 5.
Ectopic expression of CKI-2 results in stem cell loss in FBF(−) animals. (A) CKI-2 is expressed in distal-most gonads of fbf-1 fbf-2 animals. Immunodetection of CKI-2 in distal gonads (outlined), dissected at 0.5 days after the adult moult, from animals of the indicated genotypes. The proximal fbf-1 fbf-2 gonad (arrow) contains sperm and therefore does not express CKI-2. (B) Stem cell loss in fbf-1 fbf-2 mutants is largely rescued by removing cki-2. Top: Distal-most cells of fbf-1 fbf-2 mutant gonads dissected at 1 day after the adult moult and stained for the indicated markers. Stem cells do not express the meiotic marker HTP-3, and those in mitosis are positive for H3S10p staining which marks condensed chromosomes. Conversely, loss of H3S10p staining and expression of HTP3 indicates meiotic entry. Nuclei of germ cells that differentiated into sperm have a characteristic dot-like appearance visualized by DAPI staining. Bottom: Fractions of gonads that in the distal-most part contain: stem cells (light blue), meiotic cells (intermediate blue), or sperm (dark blue), measured at 12 h intervals after the larval-to-adult moult. (C) Depletion of CKI-2 from fbf-1 fbf-2 gonads partially restores stem cell proliferation. Numbers of H3S10p-positive (mitotic) cells in distal-most gonads from animals of indicated genotypes are shown. Number of gonads analysed for panels (B) and (C) fbf-1 fbf-2/fbf-1 fbf-2 cki-2/wild type: d0=58/45/44, d0.5=43/28/48, d1=41/43/13, d1.5=7/34/31, d2=43/19/44.
Discussion
Several members of the PUF protein family have been associated with stem cell self-renewal in diverse organisms (Wickens et al, 2002). Like many RNA-binding proteins, FBF associates with, and presumably regulates, hundreds of mRNAs (Kershner and Kimble, 2010). Apart from cki-2, its targets include mRNAs encoding structural components of meiotic chromosomes such as HIM-3, HTP-1 and -2, SYP-1 and SYP-2, which are ectopically expressed in FBF(−) gonads (Kershner and Kimble, 2010; Merritt and Seydoux, 2010). However, in contrast to CKI-2, depletion of those meiotic proteins does not restore stem cells in fbf-1 fbf-2 mutants (Merritt and Seydoux, 2010). This suggests that cki-2 mRNA is the key target of FBF in stem cell maintenance.
Though cki-2 deletion has no obvious consequences for germ cell development, our findings suggest that CKI-2 repression in GSCs is critical for stem cell maintenance. This agrees with observations that cell-cycle inhibitors, including Cip/Kip family members, are usually depleted from stem cells (Stead et al, 2002; Ginis et al, 2004). The regulation of Cip/Kip mRNAs by PUF proteins appears to be evolutionarily conserved (Morris et al, 2008). A vertebrate PUF protein, PUM1, binds to mRNA encoding p27, allowing miRNA-mediated repression (Kedde et al, 2010). However, cki-2 is a weak candidate for miRNA-mediated regulation based on the available prediction tools (MirWip, PicTar, TargetScan and MirZ; our observation). Only the TargetScan software identified the (non-conserved) seed region of miRNAs 44, 45, 247 and 61 (CUAGUCA) in the cki-2 3′UTR. However, the Δ1 deletion removes this site without affecting distal repression, indicating that these miRNAs are not essential for CKI-2 regulation in the germline. On the other hand, PUF proteins have been shown to repress mRNAs by recruiting deadenylases (Goldstrohm and Wickens, 2008). In C. elegans, at least one mRNA, gld-1, appears to be repressed by FBF in a similar manner (Suh et al, 2009), suggesting a possible mechanism for cki-2 regulation. Somewhat consistently with this possibility, the cki-2 message is less abundant in GSCs (this study and Kim and Roy, 2006).
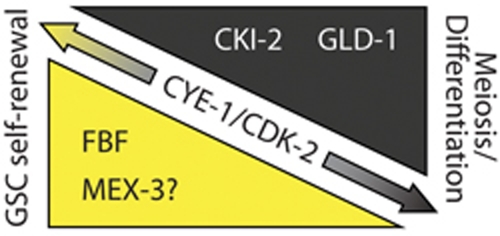
Cip/Kip CKIs are well-established inhibitors of Cyclin E/Cdk2 activity. Therefore, repression of CKI-2 in GSCs (this study), and of CYE-1/Cyclin E in differentiating cells (Biedermann et al, 2009), is expected to produce a high-low gradient of CYE-1/CDK-2 activity in the germline, which could be important for the self-renewal versus differentiation decision (Figure 6). Consistently with this model, proliferation of GSCs is sensitive to CYE-1 and CDK-2 levels (Seydoux et al, 1993; Fox et al, 2011; Jeong et al, 2011). Although this work is focused on regulation by RNA-binding proteins, other mechanisms may contribute. For example, CKIs are subject to proteasomal degradation (Lu and Hunter, 2010), and depletion of the C. elegans E3 ligase CUL-2 (Feng et al, 1999) or core proteasome components (our unpublished observation) causes proliferation defects that may involve stabilization of CKIs.
Figure 6.
Factors influencing the self-renewal versus differentiation decision of GSCs converge on the regulation of Cyclin E/Cdk2 activity. A model for CYE-1/CDK-2 regulation in the adult germline and its effect on the self-renewal versus differentiation decision. FBF and possibly MEX-3 repress the CYE-1/CDK-2 inhibitor CKI-2 in stem cells. In cells undergoing meiosis, GLD-1 represses translation of CYE-1 and may cooperate with CKI-2 in repressing CYE-1/CDK-2 activity, promoting meiosis and differentiation.
Interestingly, murine Cyclin E and Cdk2, which are mostly dispensable for development, have critical functions in the mouse germline (Geng et al, 2003; Ortega et al, 2003). Conversely, mitotic arrest and differentiation of germ cells in the fetal testes correlate with suppression of Cyclin E and activation of several CKIs, including p27Kip1 (Western et al, 2008). Also, accumulation of p27, achieved by protection of p27 mRNA from miRNA-mediated repression through the RNA-binding protein DND1 (Kedde et al, 2007), is thought to facilitate cell-cycle arrest and differentiation of spermatogenic cells (Cook et al, 2011). Thus, Cyclin E/Cdk2 emerges as an important player in germ cell development. Although the direct role of Cyclin E/Cdk2 in the self-renewal versus differentiation decision in the mammalian germline remains to be tested, it is intriguing that Cdk2 has been implicated in the self-renewal of embryonic stem (ES) cells (Neganova et al, 2009). This might reflect an intimate connection between Cyclin E/Cdk2 and self-renewal in general, or a shared mechanism operating in germ cells and early embryonic cells, from which ES cells are derived.
Materials and methods
Nematode culture, mutants, constructs and transgenic lines
Standard procedures were used to maintain animals and perform RNAi by feeding. Worms were grown at 25°C unless stated otherwise. Alleles used were mex-3(or20), gld-2(q492), gld-1(q485), glp-4(bn2)ts (I); fbf-1(ok91), fbf-2(q704), cki-2(ok2105) (II), glp-1(q175), glp-1(ar202)ts, glp-1(e2141)ts (III). Unless stated otherwise, all mutations are, or phenocopy, null alleles.
The cki-2 cDNA was determined by 3′RACE using SL1-complementary forward primers. Transgenic lines were generated using the Gateway System (Merritt et al, 2008) and the MosSCI direct insertion protocol (Frøkjaer-Jensen et al, 2008). For coordinates of deletions, see Supplementary Table S2.
Immunofluorescence and microscopy
Immunofluorescence was performed as described previously (Lin et al, 1998). Antibody dilutions were 1:15 for CKI-1 and 1:20 for CKI-2 (Feng et al, 1999), 1:2000 for H3Ser10p (Upstate 06-570; Hendzel et al, 1997) and 1:200 for HTP-3 (Goodyer et al, 2008). Secondary antibodies: goat anti-mouse alexa-568, anti-rabbit alexa-568 and anti-rabbit alexa-488 were from Molecular Probes. A Zeiss AxioImager Z1 microscope equipped with AxioncamMRm REV 2 CCD camera was used to capture images. Quantification of reporter derepression in fbf-1 fbf-2 mutants: Fluorescence was quantified using ImageJ and compared with fluorescence in distal-most nuclei of wild-type animals carrying the same reporter. The P-value was calculated by t-test in R. All images subject to direct comparison were taken at identical exposure times and were processed with Adobe Photoshop CS2 in an identical manner.
RT quantitative PCR on dissected gonads and semiquantitative PCR
gld-2(q492) gld-1 (q485); glp-1(ar202) and gld-2(q492) gld-1(q485); glp-1(e2141) animals were grown at the restrictive temperature (25°C). Fifty gonads per strain were dissected for each of three replicates in M9 buffer+1 mM Levamisole and transferred to Trizol. 1.5 μg of mouse RNA was added to the sample to facilitate precipitation. RNA was extracted according to the manufacturer's instructions. cDNA was synthesized with random hexamers using the ImProm II Reverse transcription system from Promega according to the manufacturer's instructions from 1 μg RNA. Real-time PCR was performed in duplicate on 1/20 of obtained cDNA as previously described (Biedermann et al, 2009).
For semiquantification of cki-1 mRNA, RNA was extracted with Trizol from young non-gravid adults and from embryos. cDNA was produced as above from 1 μg RNA. PCR was performed on 1/200 of the cDNA reaction using FastTaq Polymerase from Roche for 30 cycles. Images were acquired with a Syngene Transilluminator and GeneSnap software and processed with Adobe Photoshop CS2.
Northern blotting
RNA was extracted with Trizol from young non-gravid adults, from glp-4 animals grown at restrictive temperature, and from cki-2 animals. 40 μg RNA was loaded per lane. The probe corresponds to SL1, 5′UTR and CDS until nucleotide 462 (exons 1–3). The DIG Northern Starter Kit from Roche was used to prepare the probe and perform hybridization according to the manufacturer's instruction, except that the blocking solution was used at 10 × concentration.
Protein purification
The PUF domain (amino acids 121–632) of FBF-2 was cloned into pGEX-6P-1 (GE Healthcare) and transformed into BL21(DE3) codon plus cells. Cultures were grown at 37°C to an OD600 of 0.4, shifted to 4°C for 15 min, induced with 0.1 mM IPTG, then incubated at 16°C overnight. Cell pellets were resuspended in lysis buffer (50 mM Tris pH 7.5, 500 mM KCl and 2 mM DTT), supplemented with one Complete Mini EDTA-free protease tablet (Roche) per 50 ml of buffer, and disrupted using a microfluidizer. Following clarification, the protein was purified by affinity chromatography using a glutathione sepharose 4B (GE Healthcare) column. To remove co-purifying nucleic acids, the column was washed with lysis buffer supplemented with 1 M KCl then exchanged into 50 mM Tris pH 7.5, 150 mM KCl, 1 mM EDTA and 2 mM DTT. The protein was eluted by incubation with Precission protease (GE Healthcare) at 4°C overnight which cleaves in between FBF and the N-terminal GST tag. Additional contaminants were removed using a HiTrap Q HP (GE Healthcare) column where purified FBF was recovered in the flow-through. MEX-3 was purified as previously described (Pagano et al, 2009).
RNA-binding assays
RNA oligonucleotides (IDT) were labelled as described by Pagano et al (2011) and MEX-3 binding assays were performed as described in Pagano et al (2009). FBF-binding reactions were performed as described in Koh et al (2011) and Wang et al (2009), with the exception that F-EMSA and FP data were collected as described in Pagano et al (2011). See Supplementary Methods for oligonucleotide sequences.
Supplementary Material
Acknowledgments
We thank Asja Moerkamp and Mathias Senften for experimental assistance; Hans R Hotz for bioinformatic support; Ed Kipreos for CKI antibodies; Christian Lehner, Iskra Katic and Susanne Finger for comments on the manuscript. Some strains were provided by the Caenorhabditis Genetics Center funded by the NIH. IK was supported by a Boehringer Ingelheim Fonds PhD fellowship; RC by a Leukemia & Lymphoma Society Special Fellow Award; SPR by NIH grant GM081422. The Friedrich Miescher Institute is supported by the Novartis Research Foundation.
Author contributions: IK, RC, BF and SR designed experiments. BF performed in vitro association assays. IK performed all other experiments. SP provided critical technical assistance. IK and RC wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ariz M, Mainpal R, Subramaniam K. (2009) C. elegans RNA-binding proteins PUF-8 and MEX-3 function redundantly to promote germline stem cell mitosis. Dev Biol 326: 295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D, Hook B, Hajarnavis A, Opperman L, Wickens M. (2005) Binding specificity and mRNA targets of a C elegans PUF protein, FBF-1. RNA 11: 447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson A, Dowdy SF, Roberts JM. (2008) CDK inhibitors: cell cycle regulators and beyond. Dev Cell 14: 159–169 [DOI] [PubMed] [Google Scholar]
- Biedermann B, Wright J, Senften M, Kalchhauser I, Sarathy G, Lee MH, Ciosk R. (2009) Translational repression of cyclin E prevents precocious mitosis and embryonic gene activation during C. elegans meiosis. Dev Cell 17: 355–364 [DOI] [PubMed] [Google Scholar]
- Buck SH, Chiu D, Saito RM. (2009) The cyclin-dependent kinase inhibitors, cki-1 and cki-2, act in overlapping but distinct pathways to control cell cycle quiescence during C. elegans development. Cell Cycle 8: 2613–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook MS, Munger SC, Nadeau JH, Capel B. (2011) Regulation of male germ cell cycle arrest and differentiation by DND1 is modulated by genetic background. Development 138: 23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden SL, Bernstein DS, Bachorik JL, Thompson BE, Gallegos M, Petcherski AG, Moulder G, Barstead R, Wickens M, Kimble J. (2002) A conserved RNA-binding protein controls germline stem cells in Caenorhabditis elegans. Nature 417: 660–663 [DOI] [PubMed] [Google Scholar]
- Feng H, Zhong W, Punkosdy G, Gu S, Zhou L, Seabolt EK, Kipreos ET. (1999) CUL-2 is required for the G1-to-S-phase transition and mitotic chromosome condensation in Caenorhabditis elegans. Nat Cell Biol 1: 486–492 [DOI] [PubMed] [Google Scholar]
- Forbes A, Lehmann R. (1998) Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells. Development 125: 679–690 [DOI] [PubMed] [Google Scholar]
- Fox PM, Vought VE, Hanazawa M, Lee MH, Maine EM, Schedl T. (2011) Cyclin E and CDK-2 regulate proliferative cell fate and cell cycle progression in the C. elegans germline. Development 138: 2223–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjaer-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen SP, Grunnet M, Jorgensen EM. (2008) Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet 40: 1375–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S, Rideout WM, Bronson RT, Gardner H, Sicinski P. (2003) Cyclin E ablation in the mouse. Cell 114: 431–443 [DOI] [PubMed] [Google Scholar]
- Ginis I, Luo Y, Miura T, Thies S, Brandenberger R, Gerecht-Nir S, Amit M, Hoke A, Carpenter MK, Itskovitz-Eldor J, Rao MS. (2004) Differences between human and mouse embryonic stem cells. Dev Biol 269: 360–380 [DOI] [PubMed] [Google Scholar]
- Goldstrohm AC, Wickens M (2008) Multifunctional deadenylase complexes diversify mRNA control. Nat Rev Mol Cell Biol 9: 337–344 [DOI] [PubMed] [Google Scholar]
- Goodyer W, Kaitna S, Couteau F, Ward JD, Boulton SJ, Zetka M. (2008) HTP-3 links DSB formation with homolog pairing and crossing over during C. elegans meiosis. Dev Cell 14: 263–274 [DOI] [PubMed] [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD (1997) Mitosis-specific phosphorylation of histone H3 initiates primarily with pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106: 348–360 [DOI] [PubMed] [Google Scholar]
- Hong Y, Roy R, Ambros V. (1998) Developmental regulation of a cyclin-dependent kinase inhibitor controls postembryonic cell cycle progression in Caenorhabditis elegans. Development 125: 3585–3597 [DOI] [PubMed] [Google Scholar]
- Jeong J, Verheyden JM, Kimble J. (2011) Cyclin E and Cdk2 control GLD-1, the mitosis/meiosis decision, and germline stem cells in Caenorhabditis elegans. PLoS Genet 7: e1001348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadyk LC, Kimble J (1998) Genetic regulation of entry into meiosis in Caenorhabditis elegans. Development 125: 1803–1813 [DOI] [PubMed] [Google Scholar]
- Kato JY, Sherr CJ (1993) Inhibition of granulocyte differentiation by G1 cyclins D2 and D3 but not D1. Proc Natl Acad Sci U S A 90: 11513–11517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedde M, Strasser MJ, Boldajipour B, Oude Vrielink JA, Slanchev K, le Sage C, Nagel R, Voorhoeve PM, van Duijse J, rom UA, Lund AH, Perrakis A, Raz E, Agami R. (2007) RNA-binding protein Dnd1 inhibits microRNA access to target mRNA. Cell 131: 1273–1286 [DOI] [PubMed] [Google Scholar]
- Kedde M, van Kouwenhove M, Zwart W, Oude Vrielink JA, Elkon R, Agami R. (2010) A Pumilio-induced RNA structure switch in p27–3′ UTR controls miR-221 and miR-222 accessibility. Nat Cell Biol 12: 1014–1020, [DOI] [PubMed] [Google Scholar]
- Kershner AM, Kimble J (2010) Genome-wide analysis of mRNA targets for Caenorhabditis elegans FBF, a conserved stem cell regulator. Proc Natl Acad Sci U S A 107: 3936–3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Roy R (2006) Cell cycle regulators control centrosome elimination during oogenesis in Caenorhabditis elegans. J Cell Biol 174: 751–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J, Crittenden SL (2007) Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu Rev Cell Dev Biol 23: 405–433 [DOI] [PubMed] [Google Scholar]
- Kodoyianni V, Maine EM, Kimble J. (1992) Molecular basis of loss-of-function mutations in the glp-1 gene of Caenorhabitis elegans. Mol Biol Cell 3: 1199–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh YY, Wang Y, Qiu C, Opperman L, Gross L, Tanaka Hall TM, Wickens M. (2011) Stacking interactions in PUF-RNA complexes. RNA 17: 718–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont LB, Crittenden SL, Bernstein D, Wickens M, Kimble J. (2004) FBF-1 and FBF-2 regulate the size of the mitotic region in the C elegans germline. Dev Cell 7: 697–707 [DOI] [PubMed] [Google Scholar]
- Lange C, Calegari F (2010) Cdks and cyclins link G(1) length and differentiation of embryonic, neural and hematopoietic stem cells. Cell Cycle 9: 1893–1900 [DOI] [PubMed] [Google Scholar]
- Lange C, Huttner WB, Calegari F. (2009) Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell 5: 320–331 [DOI] [PubMed] [Google Scholar]
- Lin R, Hill RJ, Priess JR. (1998) POP-1 and anterior-posterior fate decisions in C. elegans embryos. Cell 92: 229–239 [DOI] [PubMed] [Google Scholar]
- Lu Z, Hunter T (2010) Ubiquitylation and proteasomal degradation of the p21(Cip1), p27(Kip1) and p57(Kip2) CDK inhibitors. Cell Cycle 9: 2342–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt C, Rasoloson D, Ko D, Seydoux G. (2008) 3′ UTRs are the primary regulators of gene expression in the C. elegans germline. Curr Biol 18: 1476–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt C, Seydoux G (2010) The Puf RNA-binding proteins FBF-1 and FBF-2 inhibit the expression of synaptonemal complex proteins in germline stem cells. Development 137: 1787–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AR, Mukherjee N, Keene JD. (2008) Ribonomic analysis of human Pum1 reveals cis-trans conservation across species despite evolution of diverse mRNA target sets. Mol Cell Biol 28: 4093–4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neganova I, Zhang X, Atkinson S, Lako M. (2009) Expression and functional analysis of G1 to S regulatory components reveals an important role for CDK2 in cell cycle regulation in human embryonic stem cells. Oncogene 28: 20–30 [DOI] [PubMed] [Google Scholar]
- Orford KW, Scadden DT (2008) Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet 9: 115–128 [DOI] [PubMed] [Google Scholar]
- Ortega S, Prieto I, Odajima J, Martín A, Dubus P, Sotillo R, Barbero JL, Malumbres M, Barbacid M. (2003) Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet 35: 25–31 [DOI] [PubMed] [Google Scholar]
- Pagano JM, Clingman CC, Ryder SP. (2011) Quantitative approaches to monitor protein-nucleic acid interactions using fluorescent probes. RNA 17: 14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano JM, Farley BM, Essien KI, Ryder SP. (2009) RNA recognition by the embryonic cell fate determinant and germline totipotency factor MEX-3. Proc Natl Acad Sci U S A 106: 20252–20257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper AS, Killian DJ, Hubbard EJ. (2003) Genetic analysis of Caenorhabditis elegans glp-1 mutants suggests receptor interaction or competition. Genetics 163: 115–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priess JR, Schnabel H, Schnabel R. (1987) The glp-1 locus and cellular interactions in early C. elegans embryos. Cell 51: 601–611 [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. (2002) Stemness': transcriptional profiling of embryonic and adult stem cells. Science 298: 597–600 [DOI] [PubMed] [Google Scholar]
- Richard-Parpaillon L, Cosgrove RA, Devine C, Vernon AE, Philpott A. (2004) G1/S phase cyclin-dependent kinase overexpression perturbs early development and delays tissue-specific differentiation in Xenopus. Development 131: 2577–2586 [DOI] [PubMed] [Google Scholar]
- Salvetti A, Rossi L, Lena A, Batistoni R, Deri P, Rainaldi G, Locci MT, Evangelista M, Gremigni V. (2005) DjPum, a homologue of Drosophila Pumilio, is essential to planarian stem cell maintenance. Development 132: 1863–1874 [DOI] [PubMed] [Google Scholar]
- Seydoux G, Savage C, Greenwald I. (1993) Isolation and characterization of mutations causing abnormal eversion of the vulva in Caenorhabditis elegans. Dev Biol 157: 423–436 [DOI] [PubMed] [Google Scholar]
- Skapek SX, Rhee J, Spicer DB, Lassar AB (1995) Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science 267: 1022–1024 [DOI] [PubMed] [Google Scholar]
- Stead E, White J, Faast R, Conn S, Goldstone R, Rathjen J, Dhingra U, Rathjen P, Walker D, Dalton S. (2002) Pluripotent cell division cycles are driven by ectopic Cdk2, cyclin A/E and E2F activities. Oncogene 21: 8320–8333 [DOI] [PubMed] [Google Scholar]
- Suh N, Crittenden SL, Goldstrohm A, Hook B, Thompson B, Wickens M, Kimble J. (2009) FBF and its dual control of gld-1 expression in the Caenorhabditis elegans germline. Genetics 181: 1249–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Opperman L, Wickens M, Hall TM. (2009) Structural basis for specific recognition of multiple mRNA targets by a PUF regulatory protein. Proc Natl Acad Sci U S A 106: 20186–20191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Western PS, Miles DC, van den Bergen JA, Burton M, Sinclair AH. (2008) Dynamic regulation of mitotic arrest in fetal male germ cells. Stem Cells 26: 339–347 [DOI] [PubMed] [Google Scholar]
- Wickens M, Bernstein DS, Kimble J, Parker R. (2002) A PUF family portrait: 3′UTR regulation as a way of life. Trends Genet 18: 150–157 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.