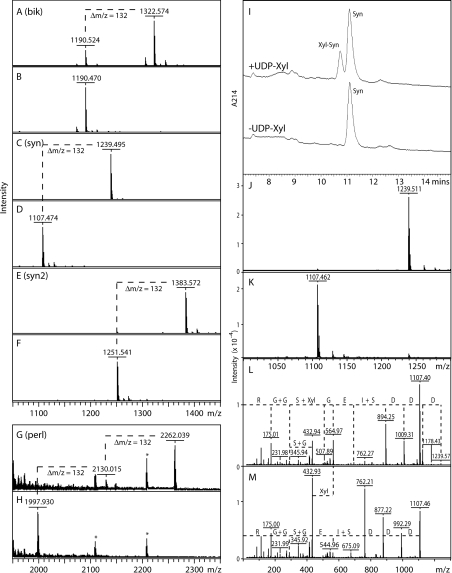
Figure 1. MALDI–TOF-MS and HPLC-based assays for xylosyltransferase activity.
(A–H) Incubation of selected peptides with the recombinant enzyme preparation was performed overnight in the presence (A,C,E,G) or absence (B,D,F,H) of UDP-Xyl as enzyme donor. In all cases, the xylosyltransferase transferred Xyl (indicated by Δm/z=132; one in the case of the Bik, Syn and Syn2 peptides, and two in the case of the Perl peptide); asterisks in (G and H) indicate contaminating peptides originating from the culture medium. (I) A typical RP-HPLC assay is depicted showing the UDP-Xyl-dependent formation over 2 h by AgOXT1 of a product with a slightly earlier elution time; product (Xyl-Syn) and substrate (Syn) peaks were collected and subject to MALDI–TOF-MS (J, K) to show the respective presence of species of m/z 1239 and 1107. The comparative MALDI–TOF-MS/MS data for the two peaks (L, M) verify the transfer of Xyl to the second serine residue of the DDDSIEGSGGR sequence of the Syn peptide (fragments of m/z 564 in D and 432 in both D and E); loss of Xyl directly from the parent ion in D is also apparent. The Δm/z values between peaks correspond to either single amino acids (D, E, G, R), dipeptides (G+G, I+S, S+G) or xylosylserine (S+Xyl).