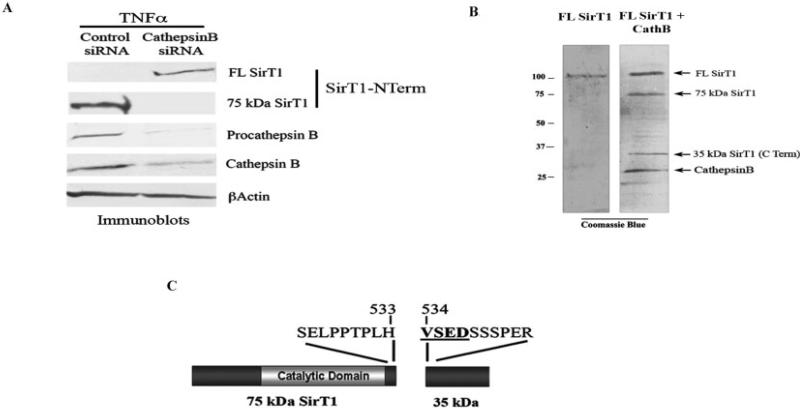
Figure 6.
Cathepsin B (CathB) cleaves SirT1 at amino acid residue 533 and is markedly elevated in osteoarthritis samples. A, Chondrocytes transiently transfected with either control small interfering RNA (siRNA) or cathepsin B siRNA were treated with tumor necrosis factor α (TNFα) for 24 hours, and cell extracts were processed for immunoblotting with the indicated antibodies. B, Commercially available SirT1 and cathepsin B were incubated in a cleavage reaction and then processed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and the gel was stained with Coomassie blue. Cathepsin B migrated to the position of the 27-kd band, as indicated. The 35-kd SirT1 fragment within the gel was excised and processed for N-terminal amino acid sequencing. Immunoblots are representative of 6 repetitions. C, N-terminal amino acid sequencing of the 35-kd SirT1 fragment. See Figure 2 for other definitions.