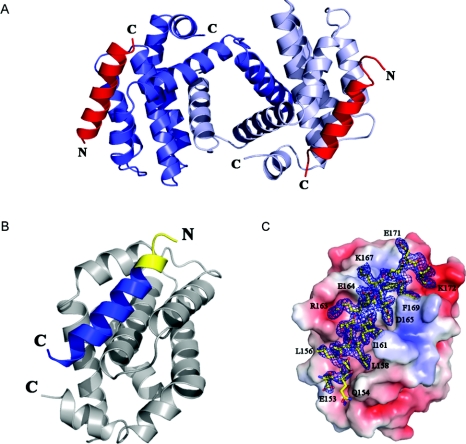
Figure 3. Crystal structure of the SOUL BH3 domain peptide complexed with Bcl-xL.
(A) A dimer of human Bcl-xL (Δ27–82) showing swapping of the α1 domains. The two helical SOUL BH3 peptides are represented in red. (B) A protomer of Bcl-xL and the SOUL BH3 peptide in contact with it. The portion of the peptide predicted by sequence homology to be the BH3 domain is in blue, whereas the yellow part is the additional portion of the peptide, the beginning of the second helix of SOUL. (C) Electron density of the SOUL BH3 peptide bound to Bcl-xL oriented as in (B). The molecular surface of Bcl-xL shows the negatively charged residues in red and those positively charged in blue. The 2Fobs−Fc map was contoured at a 1.2 σ level. Selected BH3 peptide amino acids participating in important contacts with Bcl-xL have been labelled in single-letter amino acid notation. The Figure was prepared using the program PyMOL (http://www.pymol.org).