Abstract
Acute disseminated encephalomyelitis (ADEM) is a demyelinating disease of the central nervous system (CNS) that typically presents as a monophasic disorder associated with multifocal neurologic symptoms and encephalopathy. ADEM is considered an autoimmune disorder that is triggered by an environmental stimulus in genetically susceptible individuals. The diagnosis of ADEM is based on clinical and radiological features. Most children with ADEM initially present with fever, meningeal signs, and acute encephalopathy. The level of consciousness ranges from lethargy to frank coma. Deep and subcortical white-matter lesions and gray-matter lesions such as thalami and basal ganglia on magnetic resonance imaging (MRI) are associated with ADEM. In a child who presents with signs of encephalitis, bacterial and viral meningitis or encephalitis must be ruled out. Sequential MRI is required to confirm the diagnosis of ADEM, as relapses with the appearance of new lesions on MRI may suggest either multiphasic ADEM or multiple sclerosis (MS). Pediatric MS, defined as onset of MS before the age of 16, is being increasingly recognized. MS is characterized by recurrent episodes of demyelination in the CNS separated in space and time. The McDonald criteria for diagnosis of MS include evidence from MRI and allow the clinician to make a diagnosis of clinically definite MS on the basis of the interval preceding the development of new white matter lesions, even in the absence of new clinical findings. The most important alternative diagnosis to MS is ADEM. At the initial presentation, the 2 disorders cannot be distinguished with certainty. Therefore, prolonged follow-up is needed to establish a diagnosis.
Keywords: Acute disseminated encephalomyelitis, Multiple sclerosis, Children, Child
Introduction
Acute disseminated encephalomyelitis (ADEM) is an immune mediated disease of the central nervous system (CNS) that produces multiple inflammatory lesions in the brain and spinal cord, particularly in the white matter. ADEM should be distinguished from other central inflammatory demyelinating disorders of children, including multiple sclerosis (MS) and clinically isolated syndromes that include optic neuritis, transverse myelitis, and neuromyelitis optica (Devic's disease). Most of these disorders are thought to be caused by immune system dysregulation triggered by an infectious or other environmental agent in a genetically susceptible host.
ADEM is often preceded by a viral or bacterial infection, usually in the form of a nonspecific upper respiratory infection. In 3 previous studies, an antecedent infection was identified in 72 to 77 percent of ADEM patients1-3). In general, patients will present within 1 month of this initial illness. Numerous causative pathogens have been identified to date. Viruses that have been implicated include coronavirus, coxsackie virus, cytomegalovirus, Epstein-Barr virus, herpes simplex virus, hepatitis A virus, human immunodeficiency virus, influenza virus, measles virus, rubella virus, varicella zoster virus, and West Nile virus4-11). Other organisms associated include Borrelia burgdorferi, Chlamydia, Leptospira, Mycoplasma pneumoniae, Rickettsia , and beta-hemolytic Streptococcus4,12).
Less than 5 percent of all ADEM cases follow immunization. Postvaccinal ADEM has been associated with immunization for rabies, hepatitis B, influenza, Japanese B encephalitis, diphtheria/ pertussis/tetanus, measles, mumps, rubella, pneumococcus, polio, smallpox, and varicella12). No infectious agent is isolated in most cases. Currently, measles, mumps, and rubella vaccination are most often associated with postvaccination ADEM. It is important to recognize the significant difference between the incidence of ADEM associated with the live measles vaccination (1 to 2 per million) and the incidence of ADEM previously associated with the measles virus infection (1 in 1,000)13).
The pathogenesis of ADEM is incompletely understood. The proposed mechanism of ADEM is that myelin autoantigens, such as myelin basic protein, proteolipid protein, and myelin oligodendrocyte protein, share antigenic determinants with those of an infecting pathogen12). This model is supported by studies of lymphocytes in children with ADEM. In 1 report, the frequency of T cell reactivity to myelin basic protein was 10 times higher in patients with ADEM than in those with encephalitis or normal controls5).
MS is usually thought to be a disease of young adults. However, pediatric MS, defined as onset of MS before the age of 16, is being increasingly recognized. MS presents before the age of 16 in approximately 5 percent of patients14,15). In less than 1 percent of patients, the onset of MS occurs before the age of 10 years16). In addition, pediatric MS affects girls more than boys, with a female to male ratio of 2.817). Since pediatric MS is rare, a child with recurrent episodes of acute neurologic symptoms and white matter lesions on magnetic resonance imaging (MRI) might be originally misdiagnosed with one of several other disorders, including leukodystrophies, vasculopathies, lymphoma, mitochondrial defects, and other metabolic disorders, rather than MS.
Clinical features
Neurologic symptoms of ADEM commonly appear 4 to 13 days after the infection or vaccination1-3). Fever, headache, vomiting, and meningismus are often seen at the time of initial presentation and may persist during hospitalization2,18). Encephalopathy is a characteristic feature and may progress rapidly in association with multifocal neurologic deficits4). Progression of initial neurologic signs to maximum deficits usually occurs within 4 to 7 days1,3). The level of consciousness ranges from subtle lethargy to frank coma. The altered mental status often raises concern regarding the risk of seizures, although these occur in only one-third of patients3,19).
In addition to encephalopathy, the most common neurologic features of ADEM include long tract (pyramidal tract) signs, acute hemiparesis, cerebellar ataxia, cranial neuropathies, including optic neuritis, and spinal cord dysfunction (transverse myelitis)2-4,18,19). Symptoms of optic neuritis include vision loss, pain with eye movement, and an afferent pupillary defect. Inflammation of the optic disc may be seen by direct funduscopic examination if there is extensive involvement of the optic nerve. The imaging of the optic nerve with a gadolinium-enhanced MRI of the brain and orbits is a more sensitive means to diagnose optic neuritis in these patients. Symptoms of transverse myelitis include flaccid paralysis of the legs with a change in sensory level on examination. Bowel and bladder involvement secondary to spinal cord disease results in constipation and urinary retention. The arms can be involved if the demyelinating lesion affects the cervical cord. Respiratory failure may appear with high cervical lesions that extend into the brainstem. Aphasia, movement disorders, and sensory deficits are unusual. The severe phase of ADEM generally lasts from 2 to 4 weeks. Children may show deterioration in their condition after hospital admission, and most of them develop new neurologic signs. Patients usually recover completely from the acute illness, although some have neurologic sequelae.
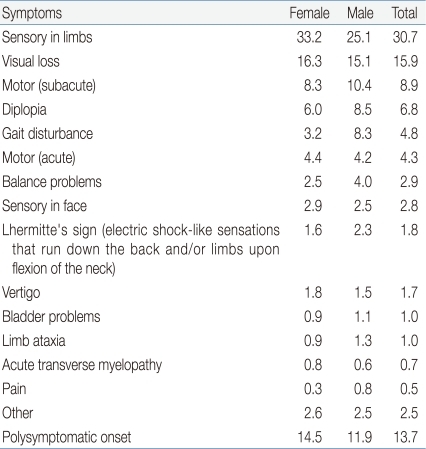
MS is a clinical diagnosis and is characterized by spatially and temporally distinct recurrent episodes of demyelination in the CNS. Acute inflammation and demyelination in a critical area of the brain, optic nerves, or spinal cord can induce a corresponding neurologic deficit. There are no clinical signs that are unique to this disorder, but some are highly characteristic (Table 1)20). Common symptoms of MS are listed in Table 220). A European observational study of 394 children with pediatric-onset MS and 1,775 patients with adult-onset MS showed that children were more likely than adults to present with isolated optic neuritis, an isolated brainstem syndrome, or symptoms of encephalopathy17).
Table 1.
Clinical Features of Multiple Sclerosis
Table 2.
Presenting Symptoms in Multiple Sclerosis
Values are presented as %.
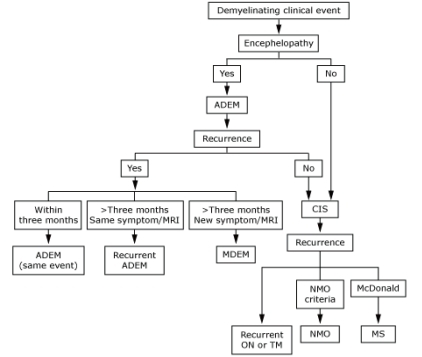
A clinically isolated syndrome (CIS) is defined as a single monosymptomatic attack compatible with MS, such as optic neuritis. An episode of CIS can produce diagnostic and therapeutic dilemmas (Fig. 1), since the vast majority of children will not have a recurrence after a single demyelinating event of the CNS. The value of examinations, including brain MRI, cerebrospinal fluid (CSF) analyses, and other laboratory studies to identify those at high risk of recurrence, is unclear21).
Fig. 1.
Diagnosis of a demyelinating clinical event. ADEM, acute disseminated encephalomyelitis; CIS, clinically isolated syndrome; MRI, magnetic resonance imaging; MDEM, multiphase disseminated encephalomyelitis; MS, multiple sclerosis; NMO, neuromyelitis optica; ON, optic neuritis; TM, transverse myelitis.
Diagnosis of ADEM
The diagnosis of ADEM is based on clinical and radiological features4). There is no specific biological marker or confirmatory test. ADEM should be suspected in a child who develops multifocal neurologic abnormalities with encephalopathy (e.g., confusion, excessive irritability, or an altered level of consciousness), especially if onset occurs one to two weeks after a viral infection or vaccination.
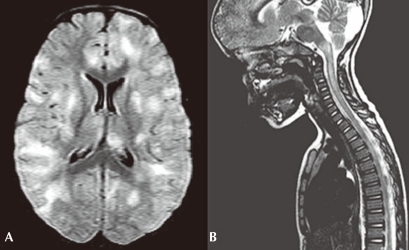
The MRI abnormalities seen in ADEM are best defined using T2-weighted images and fluid-attenuated inversion recovery (FLAIR) sequences (Fig. 2). Contrast enhancement is sometimes seen in acute lesions. Lesions associated with ADEM are typically bilateral but may be asymmetric and tend to be poorly marginated. Almost all patients have multiple lesions in the deep and subcortical white matter, while the periventricular white matter is generally spared. The thalami and basal ganglia are frequently affected (Fig. 2), and lesions in these locations are often symmetrical3,22). Brainstem and spinal cord abnormalities on MRI are common in ADEM12). The number of lesions varies, and their diameters range from <5 mm to 5 cm1,3). In the spinal cord, large confluent intramedullary lesions that extend over multiple segments are common3,12), and the degree of contrast enhancement is variable. Abnormal findings on MRI may progress over a relatively short period of time, consistent with progression of the disease. Sequential imaging by MRI is sometimes required to confirm the diagnosis of ADEM, as the occurrence of relapses, with new lesions on MRI, is not compatible with a diagnosis of monophasic ADEM, and suggests that the correct diagnosis is either multiphasic ADEM or MS, based upon the clinical symptoms and neuroimaging findings4).
Fig. 2.
(A) An axial fluid-attenuated inversion recovery magnetic resonance imaging (MRI) of the brain in a child with acute disseminated encephalopmyelitis demonstrates multifocal areas of hyperintensity in both cerebral hemispheres involving cortical gray matter, centrum semiovale, and deep gray nuclei. (B) A saggital T2-weighted MRI of the spine in the same child demonstrates high signal intrinsic to the spinal cord, consistent with longitudinally extensive transverse myelitis.
Analysis of CSF reveals pleocytosis and/or increased protein concentration in the majority of patients with ADEM1,2). However, the CSF can also be normal. Oligoclonal bands in CSF are seen in some patients with ADEM, but are a nonspecific finding more often associated with multiple sclerosis. Evaluation for infectious agents includes viral cultures of samples from the throat and nasopharynx, stool, and CSF, and serologic testing for influenza, Epstein-Barr virus, herpes, varicella, mycoplasma, cytomegalovirus, and rubella. These studies are rarely positive12).
Diagnostic criteria for ADEM in children have been proposed by the International Pediatric Multiple Sclerosis Study Group (Table 3)23). The major criteria are a clinical attack of CNS demyelinating disease with acute or subacute onset, polysymptomatic neurologic features, and encephalopathy. Encephalopathy as a presenting symptom is essential for a diagnosis of ADEM, according to the Study Group criteria23). Encephalopathy is defined as including either behavioral changes, such as lethargy or irritability, or more severe alterations in the level of consciousness, such as coma. The onset of encephalopathy almost directly corresponds to the occurrence of the disease state. This feature helps to differentiate ADEM from other clinically isolated syndromes that are related to recurrence of MS and will lead to a subsequent MS diagnosis. Brain MRI criteria for ADEM diagnosis include focal or multifocal lesions predominantly involving the white matter, without evidence of previous white-matter changes.
Table 3.
Diagnostic Criteria of Acute Disseminated Encephalomyelitis (ADEM)
Encephalopathy is a required feature for the diagnosis of ADEM, but is not a typical feature of multiple sclerosis. In addition, a cerebrospinal fluid pleocytosis ≥50 white blood cells/mm can be observed in ADEM, whereas this finding is highly atypical for multiple sclerosis. CNS, central nervous system; MRI, magnetic resonance imaging; FLAIR, fluid-attenuated inversion recovery.
The diagnostic criteria can also distinguish between monophasic ADEM with early relapses, and recurrent forms of ADEM23) on the basis of the following conditions:
- A single clinical episode of ADEM, monophasic ADEM, may evolve over as long as 3 months23). Any new and fluctuating symptoms occurring within 3 months of the initial event are considered to be part of the same event. In addition, symptoms that appear during tapering of glucocorticoid therapy, or within 1 month of completing a glucocorticoid taper, are considered to be part of the same episode.
- Recurrent ADEM refers to the recurrence, 3 or more months after the first ADEM event, of the same symptoms that occurred at the time of the initial presentation. The MRI findings are similar to those seen at the initial event and are without new lesions, although there may be enlargement of the original lesions.
- Multiphasic ADEM describes recurrent disease that fulfills criteria for ADEM but involves new anatomic areas of the CNS upon recurrence. Symptoms and signs are different from those in the initial event. The MRI must show new lesions not present during the first attack and demonstrate complete or partial resolution of the lesions associated with the first ADEM episode.
Diagnosis of pediatric MS
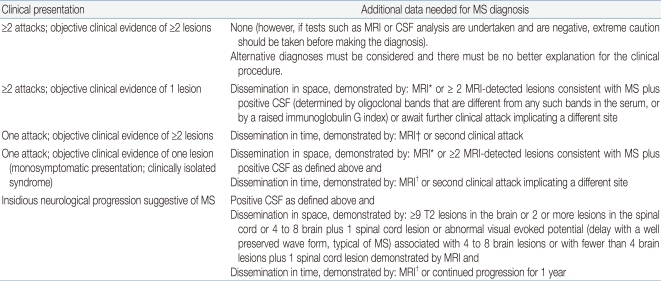
Until 2006, the Poser criteria were considered the most useful in making a diagnosis of pediatric MS24). In 2007, the Pediatric Multiple Sclerosis Study Group proposed new working definitions for pediatric MS and related demyelinating diseases such as ADEM23). The working group suggested use of the 2001 McDonald Criteria to make a diagnosis of pediatric MS25). The McDonald criteria integrate MRI findings and allow the physician to make a diagnosis of clinically definite MS based upon the time interval preceding the development of new white matter lesions, even in the absence of new clinical findings (Tables 4, 5)25,26). The McDonald criteria for dissemination in space (Table 4) specify a minimal number of lesions, with or without MRI contrast enhancement, being present in specific areas of the CNS including periventricular, juxtacortical, infratentorial, and spinal cord regions.
Table 4.
International Panel on Multiple Sclerosis (MS) Diagnostic Criteria
MRI, magnetic resonance imaging; CSF, cerebrospinal fluid; MS, multiple sclerosis.
*MRI criteria include 3 or 4 of the following: 1 gadolinium enhancing lesion or 9 T2 hyperintense lesions if there is no gadolinium enhancing lesion; at least 1 infratentorial lesion; at least 1 juxtacortical lesion; at least 3 periventricular lesions; 1 spinal cord lesion can be substituted for 1 brain lesion. †For MRI dissemination in time criteria, see Table 3.
Table 5.
International Panel on Multiple Sclerosis (MS) MRI Criteria for Dissemination in Time
MRI, magnetic resonance imaging.
The McDonald criteria were revised in 2005 for adult-onset MS26), but these revisions have not been applied to pediatric MS. A main difference between the revised McDonald criteria and the 2007 Study Group criteria for pediatric MS concerns the time required between clinical attacks or the development of new MRI lesions to qualify for the "dissemination in time." The revised McDonald criteria require a minimum of only 30 days to elapse prior to the development of a new MRI lesion to qualify as dissemination in time, while the 2007 pediatric MS criteria demand that a minimum of 3 months must pass between clinical events or the appearance of new MRI lesions23).
A characteristic pattern of MS on MRI would include multiple well-demarcated lesions in the periventricular, juxtacortical, infratentorial, and spinal cord white matter. These areas of demyelination are best recognized using T2-weighted sequences. T2 FLAIR image sequences are the most sensitive in this evaluation, especially for periventricular lesions (Fig. 3). T1-weighted sequences may reveal "black holes" that represent complete tissue loss resulting from a previous inflammatory event (Fig. 3). Enhancement of active areas of inflammation and blood-brain barrier compromise can be seen using T1 gadolinium contrast sequences. Retrospective studies suggest that children with MS onset have more T2 bright lesions in the posterior fossa and more gadolinium-enhancing lesions than adults27). Further, the lesions in children were more likely to show reversibility on follow-up MRI than lesions in adults, suggesting better recovery of demyelination in children.
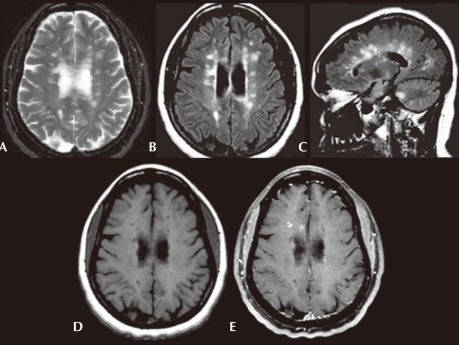
Fig. 3.
Axial T2-weighted (A) and axial fluid-attenuated inversion recovery (FLAIR) (B) images show multiple, ovoid shaped, hyperintense foci in the periventricular area, consistent with multiple sclerosis plaques. Sagittal FLAIR (C) image also shows these lesions to be radiating out from the corpus callosum. Axial precontrast T1-weighted (D) image shows that many of these lesions are hypointense, consistent with black holes. Axial postgadolinium fat saturated T1-weighted (E) image shows that some of these plaques enhance in a ring-like fashion consistent with active plaques.
Detection of increased specific immunoglobulins present only in the CSF is often useful for supporting the diagnosis of MS in adult patients. A CSF sample is considered potentially positive for MS based upon the finding of either oligoclonal immunoglobulin G (IgG) bands or an increased IgG index25). In children, the utility of CSF oligoclonal bands in MS diagnosis has been questioned1). However, positive CSF findings, when associated with new lesions on follow-up MRI, can be used to make the diagnosis of MS even in the case of a single clinical attack25).
Conclusions
In the case of a child who presents with neurologic abnormalities, including signs of encephalitis, bacterial and viral meningitis or encephalitis must be considered and ruled out. In the setting of nonspecific CSF abnormalities and identification of white-matter lesions on MRI, other inflammatory demyelinating disorders should be considered. These include ADEM, MS, optic neuritis, transverse myelitis, neuromyelitis optica, and other rare disorders. At initial onset, ADEM and MS cannot be distinguished absolutely. In children, subsequent attacks of MS may not occur for months or years. Furthermore, a small number of children with ADEM may finally develop MS, but it is difficult to accurately predict in which patients this will occur. Therefore, prolonged follow-up is required to establish a diagnosis.
References
- 1.Dale RC, de Sousa C, Chong WK, Cox TC, Harding B, Neville BG. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain. 2000;123(Pt 12):2407–2422. doi: 10.1093/brain/123.12.2407. [DOI] [PubMed] [Google Scholar]
- 2.Hynson JL, Kornberg AJ, Coleman LT, Shield L, Harvey AS, Kean MJ. Clinical and neuroradiologic features of acute disseminated encephalomyelitis in children. Neurology. 2001;56:1308–1312. doi: 10.1212/wnl.56.10.1308. [DOI] [PubMed] [Google Scholar]
- 3.Tenembaum S, Chamoles N, Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology. 2002;59:1224–1231. doi: 10.1212/wnl.59.8.1224. [DOI] [PubMed] [Google Scholar]
- 4.Tenembaum S, Chitnis T, Ness J, Hahn JS International Pediatric MS Study Group. Acute disseminated encephalomyelitis. Neurology. 2007;68(16 Suppl 2):S23–S36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- 5.Pohl-Koppe A, Burchett SK, Thiele EA, Hafler DA. Myelin basic protein reactive Th2 T cells are found in acute disseminated encephalomyelitis. J Neuroimmunol. 1998;91:19–27. doi: 10.1016/s0165-5728(98)00125-8. [DOI] [PubMed] [Google Scholar]
- 6.Stüve O, Zamvil SS. Pathogenesis, diagnosis, and treatment of acute disseminated encephalomyelitis. Curr Opin Neurol. 1999;12:395–401. doi: 10.1097/00019052-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Caldemeyer KS, Smith RR, Harris TM, Edwards MK. MRI in acute disseminated encephalomyelitis. Neuroradiology. 1994;36:216–220. doi: 10.1007/BF00588134. [DOI] [PubMed] [Google Scholar]
- 8.Kim SC, Jang HJ, Han DJ. Acute disseminated encephalomyelitis after renal transplantation in patients with positive Epstein-Barr virus antibody. Transplant Proc. 1998;30:3139. doi: 10.1016/s0041-1345(98)00967-1. [DOI] [PubMed] [Google Scholar]
- 9.Apak RA, Köse G, Anlar B, Turanli G, Topaloğlu H, Ozdirim E. Acute disseminated encephalomyelitis in childhood: report of 10 cases. J Child Neurol. 1999;14:198–201. doi: 10.1177/088307389901400312. [DOI] [PubMed] [Google Scholar]
- 10.Huber S, Kappos L, Fuhr P, Wetzel S, Steck AJ. Combined acute disseminated encephalomyelitis and acute motor axonal neuropathy after vaccination for hepatitis A and infection with Campylobacter jejuni. J Neurol. 1999;246:1204–1206. doi: 10.1007/s004150050546. [DOI] [PubMed] [Google Scholar]
- 11.Murthy JM. MRI in acute disseminated encephalomyelitis following Semple antirabies vaccine. Neuroradiology. 1998;40:420–423. doi: 10.1007/s002340050615. [DOI] [PubMed] [Google Scholar]
- 12.Stonehouse M, Gupte G, Wassmer E, Whitehouse WP. Acute disseminated encephalomyelitis: recognition in the hands of general paediatricians. Arch Dis Child. 2003;88:122–124. doi: 10.1136/adc.88.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenichel GM. Neurological complications of immunization. Ann Neurol. 1982;12:119–128. doi: 10.1002/ana.410120202. [DOI] [PubMed] [Google Scholar]
- 14.Boiko A, Vorobeychik G, Paty D, Devonshire V, Sadovnick D University of British Columbia MS Clinic Neurologists. Early onset multiple sclerosis: a longitudinal study. Neurology. 2002;59:1006–1010. doi: 10.1212/wnl.59.7.1006. [DOI] [PubMed] [Google Scholar]
- 15.Duquette P, Murray TJ, Pleines J, Ebers GC, Sadovnick D, Weldon P, et al. Multiple sclerosis in childhood: clinical profile in 125 patients. J Pediatr. 1987;111:359–363. doi: 10.1016/s0022-3476(87)80454-7. [DOI] [PubMed] [Google Scholar]
- 16.Gadoth N. Multiple sclerosis in children. Brain Dev. 2003;25:229–232. doi: 10.1016/s0387-7604(03)00035-4. [DOI] [PubMed] [Google Scholar]
- 17.Renoux C, Vukusic S, Mikaeloff Y, Edan G, Clanet M, Dubois B, et al. Natural history of multiple sclerosis with childhood onset. N Engl J Med. 2007;356:2603–2613. doi: 10.1056/NEJMoa067597. [DOI] [PubMed] [Google Scholar]
- 18.Brass SD, Caramanos Z, Santos C, Dilenge ME, Lapierre Y, Rosenblatt B. Multiple sclerosis vs acute disseminated encephalomyelitis in childhood. Pediatr Neurol. 2003;29:227–231. doi: 10.1016/s0887-8994(03)00235-2. [DOI] [PubMed] [Google Scholar]
- 19.Davis LE, Booss J. Acute disseminated encephalomyelitis in children: a changing picture. Pediatr Infect Dis J. 2003;22:829–831. doi: 10.1097/01.inf.0000087847.37363.78. [DOI] [PubMed] [Google Scholar]
- 20.Paty D, Studney D, Redekop K, Lublin F. MS COSTAR: a computerized patient record adapted for clinical research purposes. Ann Neurol. 1994;36(Suppl):S134–S135. doi: 10.1002/ana.410360732. [DOI] [PubMed] [Google Scholar]
- 21.Neuteboom RF, Boon M, Catsman Berrevoets CE, Vles JS, Gooskens RH, Stroink H, et al. Prognostic factors after a first attack of inflammatory CNS demyelination in children. Neurology. 2008;71:967–973. doi: 10.1212/01.wnl.0000316193.89691.e1. [DOI] [PubMed] [Google Scholar]
- 22.Baum PA, Barkovich AJ, Koch TK, Berg BO. Deep gray matter involvement in children with acute disseminated encephalomyelitis. AJNR Am J Neuroradiol. 1994;15:1275–1283. [PMC free article] [PubMed] [Google Scholar]
- 23.Krupp LB, Banwell B, Tenembaum S International Pediatric MS Study Group. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68(16 Suppl 2):S7–S12. doi: 10.1212/01.wnl.0000259422.44235.a8. [DOI] [PubMed] [Google Scholar]
- 24.Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 25.McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 26.Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 27.Waubant E, Chabas D, Okuda DT, Glenn O, Mowry E, Henry RG, et al. Difference in disease burden and activity in pediatric patients on brain magnetic resonance imaging at time of multiple sclerosis onset vs adults. Arch Neurol. 2009;66:967–971. doi: 10.1001/archneurol.2009.135. [DOI] [PubMed] [Google Scholar]