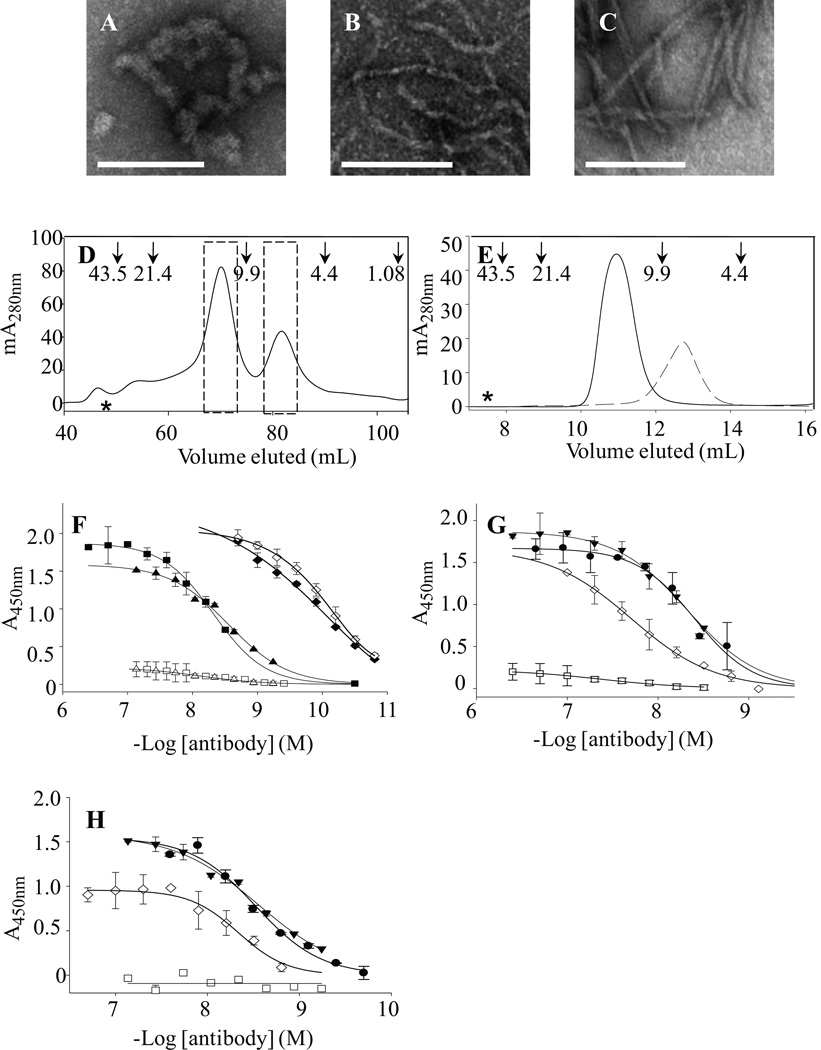
Figure 2. Aβ dimer aggregation, conformer purification, and antibody binding.
Negative contrast EM was performed on a 10 µM (Aβ1-40S26C)2 solution that was stored at −80 °C for 1 month in 25 mM ammonium acetate, pH 8.5 (A), and after 3 d at 37 °C in 20 mM sodium phosphate, pH 7.4 (B). For comparison, fibrils formed by incubating 30 µM monomeric WT Aβ for two weeks are also shown (C). Size bars in Panels A–C are 100 nm. (D) Cross-linked dimer and unreacted monomer from oxidized Aβ1-40S26C were isolated by SEC using a HiLoad 16/60 Superdex™ 75 column eluted with 25 mM ammonium acetate, pH 8.5. The dashed bars indicate the dimeric and monomeric fractions that were separately pooled and used in further experiments. Arrows indicate elution of unbranched dextran standards, and the hash symbol represents the void volume, which was determined using blue dextran 2000. (E) Rechromatographing of SEC-isolated (Aβ1-40S26C)2 ( ) and Aβ1-40S26C (
) and Aβ1-40S26C ( ) samples on a Superdex™ 75 10/300-GL column eluted with 25 mM ammonium acetate, pH 8.5, confirmed their high purity and the absence of higher molecular weight Aβ assemblies. (F) Antibody binding curves for purified IgMκ's 3C6 (■, □) and 4B5 (▲,Δ), and control mAb 6E10 (♦, ◊) against plate-immobilized freshly isolated (Aβ1-40S26C)2 (closed symbols) and Aβ1-40S26C (open symbols). Antibody binding curves were also determined for 3C6 (G) and 4B5 (H) binding to: freshly-isolated (Aβ1-40S26C)2 (●); (Aβ1-40S26C)2 stored for ~1 month at −80 °C in 25 mM ammonium acetate, pH 8.5, (▼); Freshly-isolated Aβ1-40S26C monomers (□), and WT Aβ1-40 fibrils (◊). Antibody binding studies were carried out in PBSA containing 1% BSA and 0.05% Tween 20, pH 7.4.
) samples on a Superdex™ 75 10/300-GL column eluted with 25 mM ammonium acetate, pH 8.5, confirmed their high purity and the absence of higher molecular weight Aβ assemblies. (F) Antibody binding curves for purified IgMκ's 3C6 (■, □) and 4B5 (▲,Δ), and control mAb 6E10 (♦, ◊) against plate-immobilized freshly isolated (Aβ1-40S26C)2 (closed symbols) and Aβ1-40S26C (open symbols). Antibody binding curves were also determined for 3C6 (G) and 4B5 (H) binding to: freshly-isolated (Aβ1-40S26C)2 (●); (Aβ1-40S26C)2 stored for ~1 month at −80 °C in 25 mM ammonium acetate, pH 8.5, (▼); Freshly-isolated Aβ1-40S26C monomers (□), and WT Aβ1-40 fibrils (◊). Antibody binding studies were carried out in PBSA containing 1% BSA and 0.05% Tween 20, pH 7.4.