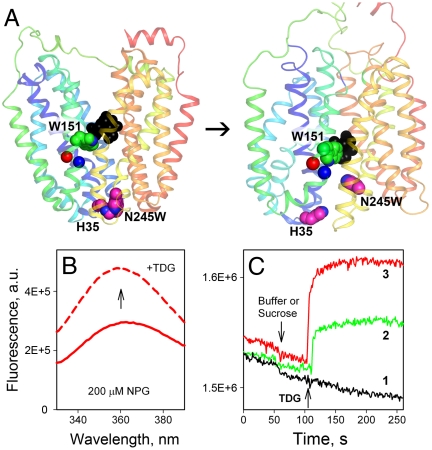
Fig. 1.
Trp fluorescence as a probe for direct measurement of sugar binding or opening of the periplasmic cavity in LacY. (A) Side view of the LacY in the inward-facing conformation (PDB ID 1PV7) is shown on the left, and the modeled outward-facing conformation (12) is shown on the right. Transmembrane helices are rainbow colored from blue (helix I) to red (helix XII). The arrow indicates the conformational transition resulting in opening of periplasmic cavity and closing of the cytoplasmic cavity. Bound sugar (black spheres) is in close proximity to Trp151 (green spheres). Trp245 introduced in place of Asn245 (helix VII) and its quencher His35 (helix I), are shown as pink spheres. Red and blue spheres represent Cα atoms at positions 154 and 155 on helix V, respectively. The figure was prepared with PyMOL 1.3. (B) Trp151-NPG FRET measured as increase of Trp fluorescence measured after displacement of bound NPG with excess TDG. Trp emission spectra of N245W/A155C LacY mutant recorded at pH 7.5 in the presence of 200 μM NPG before (red solid line) and after (red dashed line) addition of 10 mM TDG. (C) Unquenching of Trp245 fluorescence results from opening of periplasmic cavity after galactoside binding. Time courses of fluorescence changes in WT LacY (1), N245W mutant (2), or N245W/A155C mutant (3) are recorded at pH 6. Arrows indicate addition of 8 mM sucrose or TDG.