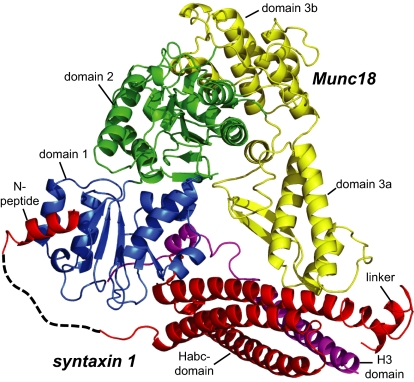
Fig. 5.
Crystal structure of the Munc18/syntaxin 1 complex from M. brevicollis. Munc18 domain 1 is formed by residues 1–129, domain 2 by residues 130–237 and 477–616, and domain 3 by residues 238–476. Domain 3 can be further subdivided into a lower half (3a) and an upper half (3b). The domains of Munc18 are colored blue, green, and yellow, respectively. The N-peptide (residues 2–15), the Habc (residues 39–172), the linker helix (183-192) of syntaxin 1 are colored red, and the H3 region (residues 210–261) is colored purple. The dashed line represents residues 16–38 of syntaxin, which were not visible in the electron density maps. Crystallographic data and refinement statistics are given in Table S1. Note that the ordered region of the bound N-peptide of syntaxin 1 is slightly longer in M. brevicollis than in the Munc18-1/syntaxin 1a structure. A more detailed description of the N-peptide is given in Fig. S5.