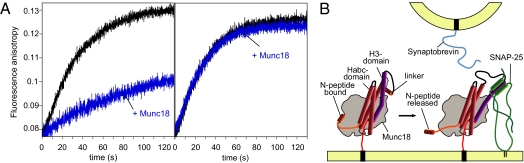
Fig. 6.
Munc18 from M. brevicollis controls SNARE assembly. Synaptobrevin was labeled with Texas red at Cys58, corresponding to Cys79 of rat synaptobrevin 2 used in our previous study (23). Ternary SNARE complex formation was followed by the increase in fluorescence anisotropy of 50 nM fluorescent Syb1-75 upon mixing with 1 μM syntaxin 1 and 3 μM SNAP-25. In the absence of Munc18, SNARE complexes for both syntaxin 1 variants, Syx1 (1-279) and Syx1 (20-279) formed (A, black curves). In the presence of Munc18 (1 μM), SNARE assembly was almost completely inhibited for Syx 1 (1–279) (Left), but not for Syx1 (20–279) (Right). We noted that the slowdown of SNARE complex formation in the presence of Munc18-1 was not found when a semiquantitative binding assay was used (31). Therefore, it needs to be emphasized here that a kinetic approach is essential to uncover the switch mechanism, whereas binding assays, by nature, are often only suited to uncover an end-product of a binding reaction. (B) Schematic model of how the release of the N-peptide might set off a conformational change that allows binding of SNAP-25 to the Munc18/syntaxin 1 complex, in turn establishing a binding site for the vesicular synaptobrevin.