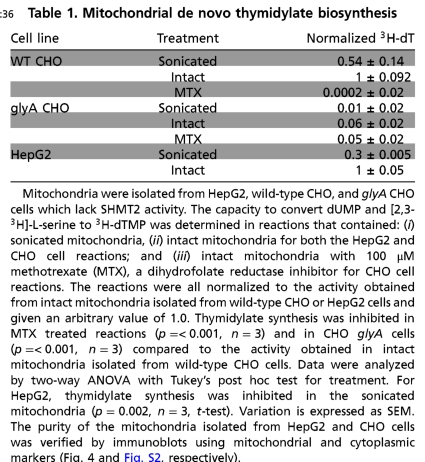
Table 1.
Mitochondrial de novo thymidylate biosynthesis
Mitochondria were isolated from HepG2, wild-type CHO, and glyA CHO cells which lack SHMT2 activity. The capacity to convert dUMP and [2,3-3H]-L-serine to 3H-dTMP was determined in reactions that contained: (i) sonicated mitochondria, (ii) intact mitochondria for both the HepG2 and CHO cell reactions; and (iii) intact mitochondria with 100 μM methotrexate (MTX), a dihydrofolate reductase inhibitor for CHO cell reactions. The reactions were all normalized to the activity obtained from intact mitochondria isolated from wild-type CHO or HepG2 cells and given an arbitrary value of 1.0. Thymidylate synthesis was inhibited in MTX treated reactions (p = < 0.001, n = 3) and in CHO glyA cells (p = < 0.001, n = 3) compared to the activity obtained in intact mitochondria isolated from wild-type CHO cells. Data were analyzed by two-way ANOVA with Tukey’s post hoc test for treatment. For HepG2, thymidylate synthesis was inhibited in the sonicated mitochondria (p = 0.002, n = 3, t-test). Variation is expressed as SEM. The purity of the mitochondria isolated from HepG2 and CHO cells was verified by immunoblots using mitochondrial and cytoplasmic markers (Fig. 4 and Fig. S2, respectively).