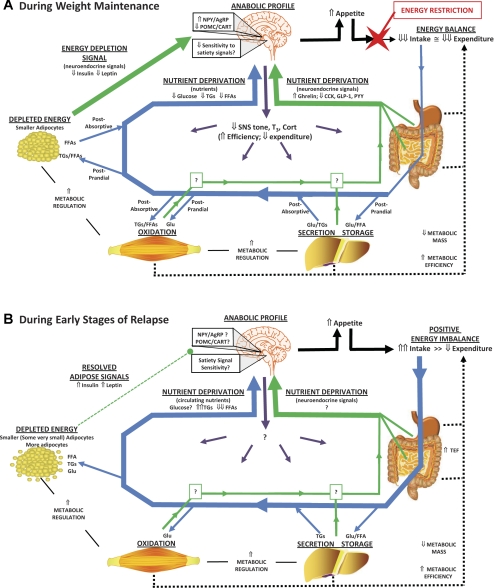
Fig. 3.
Homeostatic adaptations to an energy-restricted, low-fat diet. A: adaptations in the homeostatic system that are observed during weight maintenance, while in energy balance, are summarized. Neuroendocrine signals from the periphery (green arrows) convey a message of energy depletion (low leptin and insulin) and low nutrient availability (favoring signals of hunger over satiety/satiation) to the brain. Trafficking of absorbed nutrients (glucose, Glu; free fatty acids, FFA; triglycerides, TGs) to and from circulation is shown for both postprandial and postabsorptive metabolic states (blue arrows). Enhanced nutrient clearance reduces postprandial excursions in Glu and TGs and potentiates the postprandial suppression of FFAs, which may also convey a signal of nutrient deprivation to the brain. The signals of energy depletion and nutrient deprivation create an “anabolic” neural profile in the hypothalamus and hindbrain, increasing appetite (solid black arrows) and sending efferent signals to enhance metabolic efficiency in peripheral tissues (purple arrows). Both the reduced metabolic mass and enhanced metabolic efficiency reduce expended energy (dotted black lines). A large energy gap is created between appetite and expenditure, and food intake must be cognitively (in humans) or forcefully (in animals) restricted to maintain the reduced weight. B: when overfeeding occurs, a persistent postprandial state is created and the traditional signals of energy depletion resolve. Glu becomes the primary fuel for energy production, and the rate of clearance of all nutrients is maximized. This enhanced rate of nutrient clearance may be potentiated in some cases by the formation of very small adipocytes. Glu and TG levels in circulation become a function of the high rate of absorption from the gut and the high rate of clearance in peripheral tissues, while FFAs levels in circulation become persistently suppressed. Little is known about how the neuroendocrine signals of nutrient status, the neural profile of the hypothalamus and hindbrain, and the efferent signals sent to the periphery change during this dynamic state of weight gain. With more food absorbed and metabolized, the suppressed energy expenditure resolves to some extent because of an increase in thermic effect of food (TEF). However, the energy gap between appetite and expended energy continues until adipose depots achieve a cell size frequency distribution profile that was present prior to weight loss. At present, circulating FFAs represent the only known signal that changes during this dynamic state of weight regain in a manner that would be permissive to continued overfeeding, while having an inverse relationship with the size of adipocytes in peripheral depots.