Abstract
Myocardial contractile dysfunction develops following trauma-hemorrhagic shock (T/HS). We have previously shown that, in a rat fixed pressure model of T/HS (mean arterial pressure of 30–35 mmHg for 90 min), mesenteric lymph duct ligation before T/HS prevented T/HS-induced myocardial contractile depression. To determine whether T/HS lymph directly alters myocardial contractility, we examined the functional effects of physiologically relevant concentrations of mesenteric lymph collected from rats undergoing trauma-sham shock (T/SS) or T/HS on both isolated cardiac myocytes and Langendorff-perfused whole hearts. Acute application of T/HS lymph (0.1–2%), but not T/SS lymph, induced dual inotropic effects on myocytes with an immediate increase in the amplitude of cell shortening (1.4 ± 0.1-fold) followed by a complete block of contraction. Similarly, T/HS lymph caused dual, positive and negative effects on cellular Ca2+ transients. These effects were associated with changes in the electrophysiological properties of cardiac myocytes; T/HS lymph initially prolonged the action potential duration (action potential duration at 90% repolarization, 3.3 ± 0.4-fold), and this was followed by a decrease in the plateau potential and membrane depolarization. Furthermore, intravenous infusion of T/HS lymph, but not T/SS lymph, caused myocardial contractile dysfunction at 24 h after injection, which mimicked actual T/HS-induced changes; left ventricular developed pressure (LVDP) and the maximal rate of LVDP rise and fall (±dP/dtmax) were decreased and inotropic response to Ca2+ was blunted. However, the contractile responsiveness to β-adrenergic receptor stimulation in the T/HS lymph-infused hearts remained unchanged. These results suggest that T/HS lymph directly causes negative inotropic effects on the myocardium and that T/HS lymph-induced changes in myocyte function are likely to contribute to the development of T/HS-induced myocardial dysfunction.
Keywords: cardiomyocytes
impaired myocardial contractile function has long been recognized as an important factor involved in acute multiple organ dysfunction following hemorrhagic shock that is often associated with trauma, trauma-hemorrhagic shock (T/HS) (12, 21, 36). Despite intensive investigation, effective therapy has been hampered by a lack of understanding of the source(s) or the exact cellular mechanisms that cause the T/HS-induced myocardial dysfunction (15, 39).
Previous experimental and clinical studies (3, 5, 11) have suggested that the stressed gastrointestinal tract (i.e., gut) plays a pivotal role in the pathogenesis of systemic inflammatory response syndrome and multiple organs. Furthermore, there is emerging evidence that gut-derived factors carried in the mesenteric lymph contribute to the development of postshock organ dysfunction and failure (19, 37). The pulmonary and cardiovascular systems are two organs particularly affected following a variety of shock states (3, 5, 11). Consistent with this notion, previous studies (26) from our laboratory have shown that, in a fixed pressure model of T/HS in rats (mean arterial pressure of 30–35 mmHg for 90 min), mesenteric lymph diversion by lymph duct ligation (LDL) before T/HS completely abrogated the acute myocardial contractile dysfunction in isolated hearts. The protective effects of LDL suggest that gut-derived factors carried in T/HS lymph trigger the acute myocardial contractile dysfunction. These results present an attractive therapeutic strategy to prevent the T/HS-induced cardiac dysfunction. However, the cellular mechanism(s) for the effects of T/HS lymph in the intact heart remain(s) unclear. Specifically, because hemorrhage is a stress on the entire cardiovascular system including respiratory, renal, and neuroendcrine systems, it is not known whether T/HS lymph alters myocardial contractile function directly or indirectly through other sequalae of hemodynamic derangements.
In this study, to better understand the mechanism(s) of T/HS-induced cardiac dysfunction, we examined the functional effects of T/HS lymph in vitro both at the cellular and tissue levels. To achieve this, mesenteric lymph was collected from rats undergoing T/HS or sham shock (T/SS) and its direct effects on left ventricular (LV) myocyte cell shortening and Ca2+ transients were tested. Because an abnormality in cardiac contraction is intricately linked to electrophysiological changes at the cellular level, we also examined the effects of T/HS or T/SS lymph on the action potential waveform. Additionally, we determined whether T/HS lymph infusion in healthy rats induces cardiac dysfunction, which mimicking the characteristics of T/HS induced contractile defects (26).
The results of the present study support the notion that T/HS lymph is sufficient to trigger acute myocardial contractile dysfunction and that T/HS lymph-induced changes in cellular contractile function are centrally involved in the development of T/HS-induced myocardial contractile defects.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats (250–350 g) were used in this study. The animals were maintained in accordance with the rules of the New Jersey Medical School Animal Care and Use Committee who approved the experiments. To obtain cellular and isolated heart data, four to eight rats were examined for each protocol. “N” represents numbers of animals, and “n” represents numbers of cells examined.
T/HS models.
The model we have utilized is a fixed pressure hemorrhage shock model combined with tissue trauma in the form of a laparotomy. We have chosen this model to better reflect the clinical setting of trauma where patients experience tissue injury plus blood loss. All surgical procedures are performed as previously described (6, 19). Briefly, rats were anesthetized with pentobarbital sodium (50 mg/kg ip) and a 4- to 5-cm midline laparotomy was performed by using sterile technique. For mesenteric lymph collection, the main efferent mesenteric lymphatic was cannulated during this time period by using Silastic tubing (0.023-mm inner diameter and 0.038-mm outer diameter) flushed with heparinized saline (10 U/ml), which was secured in place with cyanomethacrylate glue (Loctite Hartford, CT) and brought out through an incision in the right flank. The laparotomy was closed after 15 min. The femoral artery was then isolated by using aseptic techniques and cannulated with polyethylene-50 tubing (0.023-mm inner diameter and 0.038-mm outer diameter) containing heparinized saline (10 U/ml). This catheter was attached in line to a blood pressure recorder (BP-2 Blood Pressure; Columbus Instruments, Columbus, OH), which was used to monitor the animals' blood pressure continuously during the shock period. Next, aseptic cannulation of the internal jugular vein was performed. Blood was withdrawn from this catheter into a syringe. The arterial pressure was reduced to 30–35 mmHg and maintained at this level for 90 min (18) by withdrawing or reinfusing shed blood (kept at 37°C). A heating pad was used to prevent hypothermia during the shock period. At the end of the shock period, animals were resuscitated by reinfusing all of the shed blood. No other fluids were infused. For collection of control laprotomy/sham shock (T/SS) lymph, rats were anesthetized, mesenteric lymphatics were cannulated, and all vascular catheters were placed identically to the T/HS rats, but no blood was withdrawn.
Lymph collection and intravenous infusion.
The mesenteric lymph samples were collected during the 90-min shock or sham shock period and 1–3 h after the end of shock or sham shock period and pooled (6). The collected lymph was centrifuged at 10,000 rpm at 4°C for 2 min to remove cellular elements and flash frozen at −80°C. Thus lymph does not contain cellular components. The collected T/HS and T/SS lymph were then infused intravenously into naïve rats at a rate of 1 ml/h for 3 h. Approximately 3 ml of lymph were produced by rats during the entire lymph collection period. In this study, to exclude the influence of anesthesia and intravenous infusion approach, the effects of saline infusion at a rate of 1 ml/h for 3 h were also included. Langendorff-perfused isolated heart experiments were performed at 24 h after intravenous injection in four groups: 1) naïve (control), 2) saline-infused, 3) T/SS lymph-infused, and 4) T/HS lymph-infused rats.
For single cardiac myocyte experiments, we used physiologically relevant concentrations of mesenteric lymph, between 0.1 and 10% vol/vol. The concentration was based on the volume of lymph produced after T/HS and blood volume of the rat (6 ml/100 g of body wt). To maintain ionic composition in media, mesenteric lymph was dialyzed with experimental media using minidialysis units (Pierce) for 2 h (33, 34).
Isolated left ventricular myocyte experiments.
Left ventricular (LV) myocytes were isolated from normal rats as previously described (33, 34). Briefly, rats were anesthetized with pentobarbital sodium (50 m/kg ip) and the heart was quickly removed. The heart was cannulated and perfused in a retrograde fashion via the aorta by the Langendorff method for ∼3 min with a modified Ca2+-free Krebs-Henseleit solution containing the following (in mmol/l): 110 NaCl, 2.6 KCl, 1.2 MgCl2, 1.2 KH2PO4, 25 NaH2PO4, 11 glucose, 30 taurine, and 10 HEPES (pH 7.4), which was oxygenated by bubbling with 95% O2-5% CO2. Then, the heart was digested in the same solution containing collagenase (type II; 0.5 mg/ml; Worthington), hyaluronidase (0.3 mg/ml; Sigma), and BSA (1 mg/ml; Sigma) for 20 min at 37°C. At the end of the digestion, the enzyme solution was washed out with enzyme-free solution containing 0.1 mM Ca2+. The LV was cut into small pieces, and the dispersed cells were filtered through a nylon mesh (200 μm). The myocytes were centrifuged at 50 g for 1 min and resuspended in a Tyrode solution containing the following (in mmol/l): 120 NaCl, 2.6 KCl, 1.0 CaCl2, 1.0 MgCl2, 11 glucose, and 5 HEPES (pH 7.3). Cells were selected for recording only if they showed normal, rod-shaped morphology with clear striation and were quiescent in the absence of stimulation.
Cell images were continuously monitored through a ×40 objective lens and transmitted to a video camera. Myocytes contraction (%cell shortening) was measured by video edge detection, as previously described (33, 34). Briefly, isolated LV myocytes were perfused with Tyrode solution at 32°C. For the Ca2+ transient measurements, cells were loaded with 2 μM fura-2 AM at room temperature for 60 min. Intracellular free-Ca2+ was monitored as the ratio of 340–380 nm fluorescence of fura-2 using the Photoscan dual-beam spectrofluorophotometer (Photon Technology). The changes in Ca2+ transients were evaluated by direct reading of the fluorescence intensity. Cells were field stimulated at 1.0 Hz for cell shortening recordings and at 0.5 Hz for intracellular free Ca2+ measurements to achieve steady-state sarcoplasmic reticulum (SR) Ca2+ loading.
Action potentials were recorded using perforated patch technique as described previously (34) at room temperature (22–23°C) to obtain stable recordings during the application of lymph. Myocytes were bathed in a Tyrode solution, containing the following (in mmol/l): 135 NaCl, 1.8 CaCl2, 1 MgCl2,5.4 KCl, 10 glucose, and 10 HEPES (pH 7.4) The pipette solution contained amphotericin B (200 μg/ml) and the following (in mmol/l): 140 KCl, 2 MgCl2, 10 NaCl, 2 ATP, and 5 HEPES, 5 (pH 7.4).
Isolated perfused heart experiments.
Intrinsic heart function was assessed by removing the heart and perfusing it with a Krebs-Henseleit buffer as previously described (26). Briefly, 24 h after the T/HS or T/SS lymph infusion, the rats were treated with heparin (200 IU/kg ip) and anesthetized with pentobarbital sodium (50 mg/kg ip). Each heart was rapidly excised, and the ascending aorta was cannulated. The cannula was attached to a Langendorff system (ADInstruments, Colorado Springs, CO). The hearts were perfused in a retrograde fashion with Krebs-Henseleit buffer containing the following (in mmol/l): 118 NaCl, 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, and 11 glucose (pH 7.4). During the initial equilibration period, the hearts were allowed to stabilize for 10–20 min at constant LV end-diastolic (LVEDP) pressure adjusted to 5 to 10 mmHg. With the use of the roller pump, to quantify intrinsic contractile performance, each heart was exposed to increasing coronary flow rates within physiological ranges (6–12 ml/min) without pacing (13, 26, 29). For studies of Ca2+ dependent contractile responses, the perfusate was switched to solutions with different Ca2+ concentrations at the constant flow rate with a peristaltic pump until they appeared to be at steady state (for ∼1–2 min). The perfusion buffer was filtered through a 45-μm filter and continuously gassed with 95% O2-5% CO2. The whole system was water jacketed and maintained at 37°C.
LV systolic pressure and LVEDP were assessed by measuring the intraventricular pressure with a fluid-filled balloon (polyethylene film) that had been inserted into the left ventricle via the mitral valve from the left atrium. This balloon was connected to a pressure transducer (MLT 844; ADInstruments, Colorado Springs, CO). Left ventricular development pressure (LVDP) was calculated as the difference between the peak systolic pressure and LVEDP.
The maximum rates of LVDP rise (+dP/dtmax) and fall (−dP/dtmax) were obtained using an electronic differentiator. All parameters were stored and analyzed off-line using PowerLab software (ADInstruments).
Statistical analysis.
Results are expressed as mean values ± SE. Statistical analysis was performed by either t-tests or one-way ANOVA followed by Bonferroni post hoc testing for multiple comparisons. Values of P < 0.05 were considered to be statistically significant.
RESULTS
Effects of T/HS lymph on cardiac myocyte contractility and Ca2+ transients.
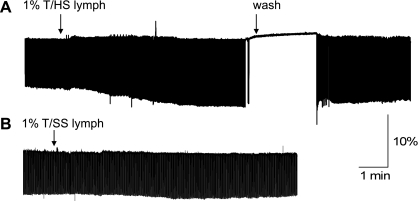
T/HS lymph at 0.1–2% produced dual, stimulatory and inhibitory inotropic effects on LV cell shortening. Figure 1A shows a representative time course of effect of T/HS lymph (1%) on cell shortening in field-stimulated myocyte (n = 16). The onset of a positive T/HS lymph action was rapid; the increase of twitch amplitude occurred 10–20 s after the application and reached a steady level within 2–3 min. The T/HS lymph-induced positive inotropic effects were followed by a loss of cellular contractile response to field stimulation. Both stimulatory and inhibitory effects occurred more rapidly with higher T/HS lymph concentrations (>1%). The T/HS lymph-induced effects were reversible upon removal of T/HS lymph (∼5–8 min). At the higher concentrations of T/HS lymph (≥ 2%), recovery of the inhibitory effects were incomplete (data not shown). It is noteworthy that the concentrations of exogenous T/HS lymph that induced changes in cell shortening fell within the theoretically calculated range for T/HS lymph levels in rats after T/HS (materials and methods).
Fig. 1.
Typical example of myocyte twitch contraction recorded in myocytes isolated from normal rat during acute application of trauma-hemorrhagic shock (T/HS) lymph (A) and control, trauma-sham shock (T/SS) lymph (B). Note: T/HS lymph caused dual, stimulatory and inhibitory (i.e., complete loss of contraction) inotropic effects on cell shortening. In contrast, there was no significant change in myocyte contraction during the application of T/SS lymph. Cell shortening was triggered by field stimulation at a frequency of 1.0 Hz.
It is also important to note that the effects observed in cardiac myocytes were achieved using mesenteric lymph dialyzed with experimental media to exclude any inotropic effects due to altered ionic composition, especially extracellular Na+, Ca2+, and K+ concentrations. Furthermore, as shown in Fig. 1B, control, T/SS lymph induced no observable change in cell shortening over 5–10 min (n = 8).
These results suggest that T/HS lymph causes a biphasic cardiac effect, a positive, early inotropic effect, as well as a delayed negative inotropic effect on isolate myocyte contraction.
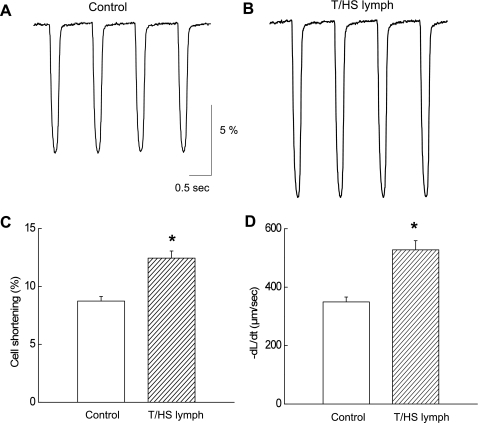
We next analyzed the characteristics of the altered contractility induced by T/HS lymph. Figure 2, A and B, shows expanded time scale showing that T/HS lymph (1%) increased the amplitude of cell shortening and rate of relaxation (cell relengthening) during stimulatory responses (2–3 min application). On the average (n = 16), T/HS lymph increased the amplitude of contraction (1.4 ± 0.2-fold; Fig. 2C) and the maximum rate of relaxation (1.54 ± 0.2-fold; Fig. 2D). It is a well-established concept that rates of contraction are associated with SR Ca2+ ATPase activity, and the increased relaxation velocity in the presence of T/HS lymph could be related to enhanced Ca2+ removal via SR (1). On the other hand, the faster rate of relaxation could have been secondary to increased amplitude of cell shortening. We therefore compared relaxation time course before and after the application of T/HS lymph.
Fig. 2.
Myocyte contraction analysis during the stimulatory effects of 1% T/HS lymph. Control (A) and in the presence of T/HS lymph (B). Pooled data for cell shortening (C; %) and rate of relaxation (D; +dL/dt) are shown. Data are expressed as means ± SE (n = 16 from 5 rats). *P < 0.05 vs. control.
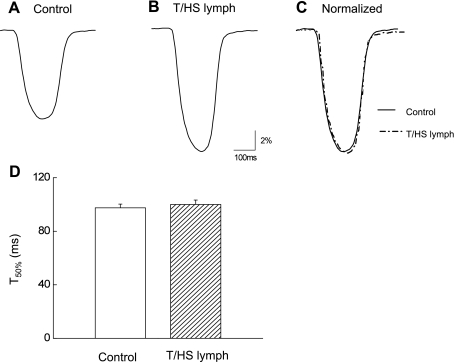
Figure 3, A and B, shows that T/HS lymph increased cell shortening. Normalized and superimposed traces (Fig. 3C) demonstrate that both contractile and relaxation function were not significantly altered by T/HS lymph. Averaged relaxation time assessed by the time for 50% decay (T50%) of cell shortening before and after the application of T/HS lymph (n = 16) are summarized in Fig. 3D. These results suggest that changes in T/HS lymph-induced positive inotropic effects are not associated with enhanced SR Ca2+ uptake function.
Fig. 3.
Left ventricular (LV) myocyte cell shortening recordings in control (A) and during stimulatory effects of T/HS lymph (B; 1%). C: traces were also normalized and superimposed to compare the time course of myocyte contraction. D: pooled data for half-time of twitch contraction (T50%). Data are expressed as means ± SE (n = 16 from 5 rats). *P < 0.05 vs. control.
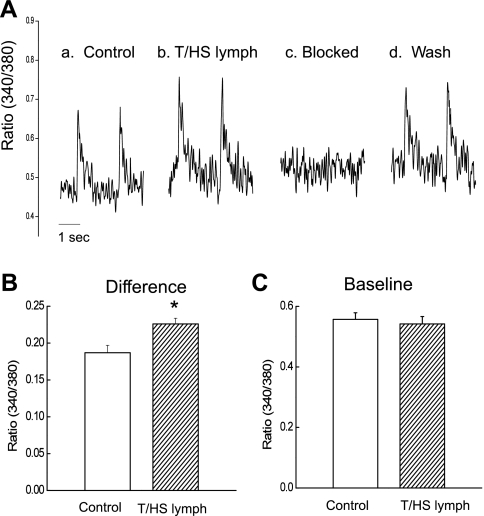
We also found that T/HS lymph induces changes in cellular Ca2+ transients (n = 8). As shown in Fig. 4A, T/HS lymph (1%) initially increased the amplitude of peak Ca2+ transient (Fig. 4, Aa and b). The initial increase of the amplitude of Ca2+ transients was from 0.187 ± 0.01 to 0.226 ± 0.01 (Fig. 4B). This was followed by a complete block of cell response to stimulation (Fig. 4Ac) and beating resumed after washout (Fig. 4Ad). There was no significant change in the diastolic, baseline Ca2+ (Fig. 4C). Control T/SS lymph at concentrations (0.1–5%) had no detectable effects on Ca2+ transients (n = 6, from 4 rats).
Fig. 4.
Effects of acute application of T/HS lymph (1%) on Ca2+ transients in LV myocytes (A). Ca2+ transients in control before (a), during stimulatory effects (b), and blocking effects (c) and after washout (d). Pooled data for difference between peak and baseline (B) and baseline in control and in the presence of T/HS lymph (C; 1%). Data are expressed as means ± SE (n = 8 from 4 rats). *P < 0.05 vs. control.
Effects of T/HS lymph on action potential profile.
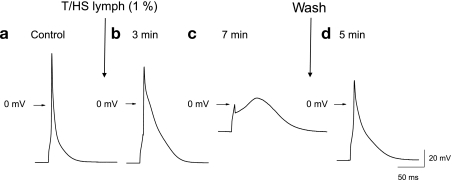
We next examined whether the above T/HS lymph-induced changes in cellular function are associated with changes in the electrophysiological properties. This was studied by measuring the action potential waveform in cardiac myocytes. Figure 5 shows a typical example of T/HS lymph (1%) effects on action potential configuration. T/HS lymph (1%) caused an immediate negative shift in plateau potential associated with marked prolonged action potential duration (Fig. 5, a and b). The overshoot potential was significantly lowered from 48.5 ± 2.3 to 16.7 ± 2.2 ms (63 ± 6%; n = 9). The action potential duration quantified at 90% repolarization was increased by 3.3 ± 0.4-fold, from 52.3 ± 6.0 to 166.9 ± 10.3 ms. The prolongation of APD was then followed by a significant rise of the resting membrane potential. As shown in Fig. 5c, after a 7- to 10-min application of T/HS lymph, the plateau potential was further reduced. After this, the cell did not repolarize and the membrane potential remained at the 0-mV level. These effects were partially reversible upon washout (3–5 min) of T/HS lymph (Fig. 5d). These results suggest that treatment of T/HS lymph leads to significant changes in the electrophysiological properties of isolated cardiac myocytes.
Fig. 5.
Typical effects of T/HS lymph (1%) on action potential recorded in LV myocytes. Action potentials recorded at different time points control before (a), during application of T/HS lymph (b and c), and after washout (d).
Effects of T/HS lymph infusion on myocardial contractile function.
Our recent studies suggested that T/HS lymph is necessary to activate this process (26). To determine whether T/HS lymph is sufficient to produce the myocardial dysfunction, we intravenously injected T/HS lymph into naïve rats and examined the contractile function at 24 h after the injection. Myocardial contractile function was assessed using the Langendorff-perfused rat heart model, because use of an isolated heart to measure myocardial contractile reserve would indicate intrinsic changes in myocardial contractile function and also enable us to compare the results with our earlier studies on the effects of T/HS (26).
Since the anesthesia and the surgery for the intravenous infusion could influence cardiac function, to confirm the specificity of the observed effects with lymph, our initial experiments compared LV contractile performance in naïve (uninstrumented) rats and in rats at 24 h after saline infusion (at a rate of 1 ml/h for 3 h). As summarized in Table 1, baseline cardiac function under physiological conditions where the hearts were perfused with Krebs-Henseleit buffer (2 mM Ca2+) at a constant coronary flow rate of 8 ml/min (29) is comparable in naïve and saline-infused groups, and the data were pooled into one group designated as the control group.
Table 1.
Baseline left ventricular function measured in naïve (control) rats and rats 24 h after saline infusion
| HR, beats/min | LVDP, mmHg | +dP/dtmax, mmHg/s | −dP/dtmax, mmHg/s | N | |
|---|---|---|---|---|---|
| Control | 238.6 ± 9.3 | 101.9 ± 6.6 | 2,226.7 ± 258 | 1,736.8 ± 136 | 4 |
| Saline | 252.9 ± 16.4 | 102.1 ± 7.2 | 2,385.0 ± 233 | 1,778.5 ± 114 | 7 |
Values are means ± SE; N = number of rats. HR, heart rate; LVDP, left ventricular developed pressure; +dP/dtmax and −dP/dtmax, LVDP rise and fall, respectively.
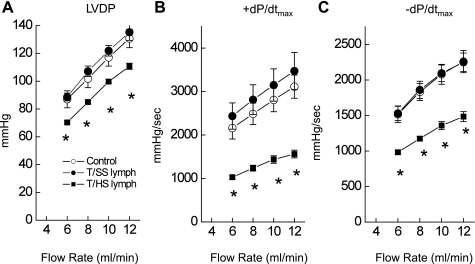
Upon infusion of T/SS lymph (N = 12), cardiac function assessed by LVDP and ± dP/dtmax showed no significant changes compared with control hearts (see also Figs. 6 and 7). In contrast, after T/HS lymph infusion, LV contractile function was significantly reduced compared with controls. Figure 6 summarizes the T/HS infusion effects on LV contractile function compared with control or T/SS lymph-infused hearts. The hearts were exposed to different coronary flow rates, and LV function was analyzed at a steady state. In control rat hearts, there was an increase in coronary perfusion pressure as flow rate was increased, but no significant change in LVEDP or heart rates. There was no difference in these values in T/HS lymph-infused hearts compared with control or T/SS lymph-infused hearts. In contrast, LVDP increased as coronary flow rates increased (Fig. 6A). Similarly, both the rise (+dP/dtmax; Fig. 6B) and fall (−dP/dtmax; Fig. 6C) of LVDP were increased with increasing flow rates. However, hearts from the T/HS lymph-infused group showed significantly lower amplitudes of LVDP, +dP/dtmax, and −dP/dtmax compared with the control group. Thus T/HS lymph infusion could result in impaired responsiveness to changes in the flow rates. In contrast, there was no change in myocardial contractility (LVDP or ± dP/dtmax) for the hearts infused with T/SS lymph compared with controls.
Fig. 6.
LV performance to increasing coronary flow rates is blunted in hearts following T/HS lymph infusion. Changes in left ventricular developed pressure (LVDP; A), +dP/dtmax (B), and −dP/dtmax (C) in response to changes in coronary flow rate. In control rat hearts, LVDP and the rates of rise (+dP/dtmax) and fall (−dP/dtmax) were increased with increasing coronary flow rates. However, the responses to increasing flow rates were depressed after the T/HS lymph infusion. Data are expressed as means ± SE from 6 rats. *P < 0.05 vs. control and T/SS lymph groups.
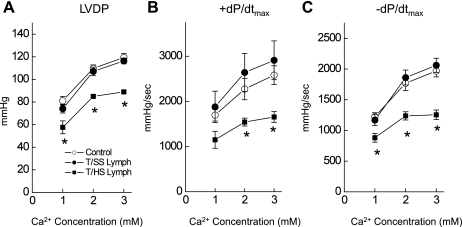
Fig. 7.
LV performance to increasing Ca2+ concentrations in the perfusate was blunted in the hearts following T/HS lymph infusion. Changes in LVDP (A), +dP/dtmax (B), and −dP/dtmax (C) in response to Ca2+ in the perfusate. In control rat hearts, LVDP and the rates of rise (+dP/dtmax) and fall (−dP/dtmax) were increased with an acute increase in Ca2+ concentration. Responses to Ca2+ were depressed in hearts with T/HS lymph infusion. Data are expressed as means ± SE from 6 rats. *P < 0.05 vs. control and T/SS lymph groups.
We also characterized the myocardial contractile response to increased extracellular Ca2+ (Fig. 7), because results from hearts following T/HS exhibited an impairment of cellular Ca2+ handling (26). Under normal physiological conditions (1–3 mM Ca2+) LVDP (Fig. 7A), +dP/dtmax (Fig. 7B) and −dP/dtmax (Fig. 7C) were increased in all groups. However, T/HS lymph-infused heart showed blunted response to Ca2+ compared with controls and T/SS lymph-infused groups, indicating impaired Ca2+ signaling pathways.
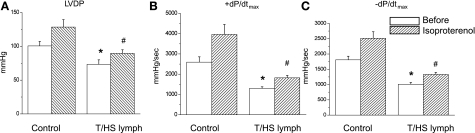
Additional experiments were performed to determine the molecular basis for the T/HS lymph-induced effects on myocardial dysfunction. The β-adrenergic receptor (β-AR) signaling system is an integral pathway in regulating myocardial contractility, particularly during stress. Depressed inotropic and lusitropic responsiveness to β-AR stimulation is noted in failing hearts (25, 32). To examine whether T/HS lymph-induced myocardial depression is secondary to uncoupling of the β-AR rather than a direct depression in basal myocardial contractility per se, we compared the effects of β-AR agonist, isoproterenol (Iso) at 10 nM on myocardial performance in control and T/HS infused hearts (Fig. 8). In the presence of Iso, LVDP (Fig. 8A) and ± dP/dt (Fig. 8, B and C) increased similarly from baseline in both control and T/HS groups. In the presence of Iso, heart rate was also increased similarly in control and T/HS lymph-infused groups by 1.35 ± 0.05- and 1.35 ± 0.02-fold, respectively. Although the relative increase in myocardial contractile function by Iso in T/HS lymph-infused hearts is not significantly different from controls, absolute values of cardiac function remained lower. Thus these results suggest that the negative inotropic effects caused by T/HS lymph infusion are unlikely mediated through β-AR uncoupling.
Fig. 8.
Assessment of LV performance t in response to the β-adrenergic receptor agonist isoproterenol (Iso). Changes in LVDP (A), +dP/dtmax (B), and −dP/dtmax (C) in response to Iso (10 nM). Administration of Iso increased inotropic performance in all groups, but cardiac performance remained significantly depressed in T/HS lymph-infused group. Data are expressed as means ± SE (n = 7 and 4 for control and T/HS lymph groups). *P < 0.05 vs. control before the application of Iso. #P < 0.05 vs. control with Iso.
DISCUSSION
During the course of previous studies, we (26) observed that mesenteric lymph diversion prevents T/HS-induced acute myocardial contractile dysfunction. We suggested that T/HS lymph exerts negative inotropic effects on the myocardium. However, several critical questions remain, e.g., given the complexity of the pathophysiological conditions of T/HS, it has not been possible to determine whether T/HS lymph has a direct effect on the cardiac myocyte itself or whether the effects occur indirectly through the altered cardiovascular function. In this study, in which the functional effects of T/HS lymph were studied systematically in the intact LV cardiac myocytes, we show that T/HS lymph directly alters cardiac myocyte contractility and intracellular Ca2+ homeostasis. The effects on cellular function were associated with changes in action potential waveform. Further studies showed that intravenous infusion of T/HS lymph into intact rats induces myocardial contractile dysfunction, which is qualitatively similar to T/HS-induced myocardial contractile dysfunction. These results provide strong support to the hypothesis that T/HS lymph triggers myocardial contractile dysfunction through a direct effect on electrophysiological and contractile properties at the cellular level.
Signaling pathways involved in the development of cardiac dysfunction after T/HS.
Previous experimental studies have proposed that cardiac depressant factor released in the systemic circulation during hemorrhagic shock contributes to loss of contractile performance. However, the occurrence of myocardial depressant effects of shock blood, shock plasma, or plasma fraction has been inconsistent and controversial (8, 10, 24). While the precise reason(s) for the disparity of earlier result is not entirely clear, it may relate, at least in part, to the method used to generate hemorrhagic shock states, e.g., length and severity of shock, with or without tissue injury (18). Furthermore, T/HS-induced alterations in preload and afterload, as well as other neuro-humoral effects, may confound the assessment of myocardial contractility (21).
More recently, with the use of well-controlled animal models of T/HS, a series of pathophysiological studies have convincingly illustrated elevation of intracellular expression of pro-inflammatory mediators such as TNF-α or interleukin IL-6 in the heart following T/HS (28, 31). Although the exact molecular signaling pathways that are responsible for the negative inotropic effects of T/HS are not known, these studies suggest that T/HS activates signaling cascades that modulates myocardial contractile function (13, 22). However, since the assessments of changes in cardiac function were conducted using whole heart measurements, the cellular basis for T/HS-induced myocardial contractile dysfunction and how the reported changes in cytokines lead to myocardial contractile depression after T/HS have not been clarified.
The cellular and molecular mechanisms for the altered myocardial contractile properties have been studied primarily in cardiac hypertrophy and failure (1, 14). These studies have shown that an abnormal cellular Ca2+ handling may underlie the depressed myocardial contractility in heart failure. Alterations in cellular Ca2+ signaling have also been proposed to play a critical role in the myocardial and vascular smooth muscle contractile dysfunction in the burn-, trauma-, and sepsis-related shock conditions (27).
The present study, in which the effects of T/HS lymph were studied in the intact LV myocytes, supports the notion that T/HS lymph-induced changes in cellular function are important initiating events (cause not consequence) that significantly contribute to myocardial contractile dysfunction. Previous experimental studies on myocyte function suggested that proinflammatory cytokines such as TNF-α and IL-1β can produce negative inotropic effects on myocardial contractility as well as negative effects on cell shortening through either nitric oxide synthase-dependent and -independent pathways (16, 35). Although possible, since the cytokine levels did not differ between the T/HS and T/SS lymph samples (4), it is not likely that cytokines in T/HS lymph act on the myocardium to directly cause the myocardial contractile dysfunction. Likewise, since the lymph is sterile, does not contain endotoxin or bacterial DNA, it is also unlikely that bacterial components are directly involved in the T/HS lymph-mediated myocardial response. However, our recent unpublished work showing that T/HS lymph-activated TLR-4 to cause lung injury and neutrophil activation suggests that nonbacteria, noncytokine, danger signals may be mediating T/HS lymph's proinflamnatory response.
One of the hallmarks of hemodynamic alterations accompanying cardiac hypertrophy and heart failure is decreased responsiveness of β-adrenergic stimulation caused by adrenergic signal abnormalities (25). Previous studies (38) have also indicated that NO is likely responsible for the uncoupling of β-adrenergic stimulation in failing hearts. In our study, we found that myocardial responsiveness to a β-adrenergic agonist, isoproterenol, was comparable in control and T/HS lymph-infused hearts. Thus our observation indicates that changes in β-adrenergic receptor abnormalities are not responsible for the early course of T/HS lymph-induced contractile depression.
Cellular mechanisms underlying the T/HS lymph-induced changes in myocyte contractility.
The key observation from the cardiac myocytes studies was that exogenously applied T/HS lymph directly alters myocyte contractility. The effects were apparent at the concentrations (0.5–5%) that might be observed in vivo, based on the volume of intestinal lymph produced as a proportion of the blood volume of the rat (6). The contractile abnormalities were associated with alterations in the electrophysiological properties of the cardiac myocytes.
In the present study, we found that T/HS lymph-induced dual inotropic effects; a positive response, followed by a negative response in LV myocytes isolated from healthy rats as measured by cell shortening. At concentrations (0.5–1%), the onset of the depressive contractile response occurred with a half-time of 3–5 min whereas a stimulatory effect occurred quickly within 20 s after the application of T/HS lymph. At the higher concentrations (≥ 2%), T/HS lymph caused rapid block of contraction. During the early stimulatory response, T/HS lymph did not alter rates of contraction and relaxation, suggesting that the positive inotropic effect of T/HS lymph did not cause changes in SR Ca2+ uptake function.
Although it is generally agreed that changes in SR Ca2+ ATPase function rather than altered SR Ca2+ release channel (ryanodine receptor) function contribute to abnormal contractile function such as cardiac hypertrophy and heart failure (2, 7), it is important to note that altered ryanodine receptor function could also contribute to the T/HS lymph-induced myocardial contractile dysfunction.
To further investigate the cellular basis for the T/HS lymph-induced alterations in cellular contractility, we examined the effects of T/HS lymph on the action potential profile. We found that T/HS lymph had an effect on excitation-contraction (E-C) coupling by changing the action potential profile. After a 2- to 3-min application of exogenous T/HS lymph, the plateau potential was reduced and action potential duration was prolonged. These changes were followed by a further decrease of the plateau potential and membrane depolarization. The effects were partially reversible. In cardiac myocytes, it is well documented that prolongation of the action potential can result in increased magnitude of Ca2+ influx via l-type Ca2+ channels. On the other hand, membrane depolarization inactivates the Ca2+ channels and blocks Ca2+ influx. Therefore, our results indicate that there are important changes in excitability of cardiac myocytes and the changes underlie the abnormal cellular contraction during the application of T/HS lymph.
Cardiac E-C coupling involves cellular Ca2+ regulation. Changes in the action potential duration and profile result from alterations in the functional expression of inward and outward ionic currents, and/or more complex changes generated by the sarcolemmal Na+/Ca2+ exchanger and Na+/K+ electrogenic pump, and the SR Ca2+ handling proteins, such as SR Ca2+ ATPase and ryanodine receptor (2, 30). Although T/HS-induced contractile dysfunction is different from pathophysiological states (e.g., cardiac hypertrophy and heart failure), changes in these cellular components may contribute to the T/HS lymph-induced myocardial contractile dysfunction. To further determine the mechanisms, it is important to define the mechanisms responsible for T/HS-induced action potential changes and the underlying ionic currents as well as more complex components using specific ionic conditions and pharmacological agents.
The findings that T/HS lymph initially increased cellular contractility are paradoxical to depressive response to T/HS, as measure by LV function. Although experimental T/HS models have clearly defined the presence of intrinsic myocardial contractile defects, cardiac output is often maintained in the clinical situation. Thus a rapid increase in myocyte contraction caused by T/HS lymph may represent a unique cellular adaptation mechanism to increase cardiac performance of the compromised hearts. Alternatively, since the effects of T/HS lymph on myocyte function was time and concentration dependent, it is possible that negative inotropic effects observed at 24 h after T/HS infusion may be the net physiological effects.
The exact chemical composition of the active components in T/HS lymph have not yet been evaluated; however, the effects we observed in cellular experiments may be attributed to the existence of two distinct (opposite) inotropic active components in T/HS lymph. An alternative possibility would be that a single active component mediates two effects, stimulatory and inhibitory, depending on the concentration.
Correlation of cellular findings with T/HS lymph-induced myocardial dysfunction.
We found that T/HS lymph directly alters cellular function. It is generally accepted that abnormal cardiac function is linked to abnormal cellular Ca2+ regulation in hypertrophy and heart failure. However, there is not substantial direct evidence for a causative role for depressed myocyte function in the initiation of T/HS-induced cardiac dysfunction. It is, therefore, important to demonstrate that T/HS lymph is sufficient to produce negative inotropic effects in vivo, supporting the hypothesis that T/HS lymph activates signaling pathways that are responsible for the acute myocardial contractile dysfunction.
In the present study, we found that intravenous injection of T/HS lymph into intact rats resulted in myocardial contractile dysfunction at 24 h after the infusion, which mimicked T/HS-induced cardiac depression in Langendorff-perfused hearts experiments: significantly decreased LVDP and the maximal rate of LVDP rise and fall (± dP/dtmax). The T/HS lymph-infused hearts had a blunted contractile response to increases in extracellular Ca2+ concentrations compared with the control hearts, indicating an impairment of Ca2+ handling. In addition, we found that myocardial contractility was depressed irrespective of whether the T/HS lymph infusion alone or, with a laparotomy, consistent with the evidence that T/HS lymph is capable of recreating T/HS-induced myocardial dysfunction. Nonetheless, although most of the animals studied showed significant depressive contractile response at 24 hrs after exogenous T/HS lymph infusion compared with controls, in some animals (N = 4 out of 16 experiments), the difference was not significant. This difference may be explained by certain animal-to-animal variations. For instance, in the present study, we infused lymph at 1 ml/h for 3 h, based on the fact that ∼3 ml of lymph was produced by rats during the entire lymph collection period. However, the total volume presumably may not exactly the same as in each animal. Given the complexity of the driving force for blood flow to all of the systemic vasculature, the time course of tissue uptake of T/HS lymph as well as variable cellular responses, each animal may respond differently. Moreover, as seen in cellular experiments, the effects of T/HS lymph on myocyte function were biphasic, e.g., time- and concentration dependent. It is thus possible that T/HS lymph induced varying degrees of myocardial depression in vivo based on the duration and concentrations. More studies on these factors (volume and the time course of effects) may help to clarify the heterogeneity of cardiac response to exogenous T/HS lymph.
One critical yet unresolved issue is the signaling mechanisms underlying T/HS-induced myocardial dysfunction. Although the cause of depressed myocardial performance following T/HS was not clearly identified, it is possible that the presence of factor(s) in T/HS lymph may activate NO/cGMP signaling pathways that regulates basal as well as antagonize cAMP-dependent L-type Ca2+ channel phosphorylation resulting in diminished cellular Ca2+ concentration (9, 20). Alternatively, T/HS lymph could also directly inhibits L-type Ca2+ channel function by modulating expression and the channel protein trafficking (17). In addition, alterations in other components of cardiac contraction such as changes in Ca2+ responsiveness of the myofilaments or degradation of troponin I (23) could also lead to electrophysiological and contractile changes observed in the present study.
In summary, the present results indicate that T/HS lymph can directly regulate E-C coupling at the cellular level, leading to an abnormal cellular contraction. Although there are limitations to the extent to which these experimental data can be extrapolated to clinical situations, understanding the cellular mechanisms outlined in the current study may help to identify a novel therapies to reduce acute cardiac dysfunction following T/HS.
GRANTS
Support for this research was provided by National Institutes of Health Grants HL-077480 and GM-059841.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Present address of M. Jiang: Department of General Surgery, The Second Affiliated Hospital, Harbin Medical University, Harbin, China.
REFERENCES
- 1. Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, The Netherlands: Kluwer Academic Publishers, 2001 [Google Scholar]
- 3. Charalambous BM, Stephens RC, Feavers IM, Montgomery HE. Role of bacterial endotoxin in chronic heart failure: the gut of the matter. Shock 28: 15–23, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Davidson MT, Deitch EA, Lu Q, Osband A, Feketeova E, Nemeth ZH, Hasko G, Xu DZ. A study of the biologic activity of trauma-hemorrhagic shock mesenteric lymph over time and the relative role of cytokines. Surgery 136: 32–41, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg 216: 117–134, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deitch EA, Adams C, Lu Q, Xu DZ. A time course study of the protective effect of mesenteric lymph duct ligation on hemorrhagic shock-induced pulmonary injury and the toxic effects of lymph from shocked rats on endothelial cell monolayer permeability. Surgery 129: 39–47, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Eisner DA, Trafford AW. Heart failure and the ryanodine receptor: does Occam's razor rule? Circ Res 91: 979–981, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Forrester JS, Amsterdam EA, Parmley WW, Sonnenblick EH, Urschel CW. Dissociation of myocardial contractility and pump performance in hemorrhagic shock. Correlation of in vivo measurements with assay of shock plasma in papillary muscle. Cardiology 57: 333–347, 1972 [DOI] [PubMed] [Google Scholar]
- 9. Gallo MP, Malan D, Bedendi I, Biasin C, Alloatti G, Levi RC. Regulation of cardiac calcium current by NO and cGMP-modulating agents. Pflügers Arch 441: 621–628, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Hallstrom S, Vogl C, Redl H, Schlag G. Net inotropic plasma activity in canine hypovolemic traumatic shock: low molecular weight plasma fraction after prolonged hypotension depresses cardiac muscle performance in vitro. Circ Shock 30: 129–144, 1990 [PubMed] [Google Scholar]
- 11. Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock 15: 1–10, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Horton JW. Hemorrhagic shock depresses myocardial contractile function in the guinea pig. Circ Shock 28: 23–35, 1989 [PubMed] [Google Scholar]
- 13. Horton JW. Left ventricular contractile dysfunction as a complication of thermal injury. Shock 22: 495–507, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Houser SR, Piacentino V, III, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol 32: 1595–1607, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Hsu JT, Kan WH, Hsieh CH, Choudhry MA, Bland KI, Chaudry IH. Mechanism of salutary effects of estrogen on cardiac function following trauma-hemorrhage: Akt-dependent HO-1 up-regulation. Crit Care Med 37: 2338–2344, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Kumar A, Brar R, Wang P, Dee L, Skorupa G, Khadour F, Schulz R, Parrillo JE. Role of nitric oxide and cGMP in human septic serum-induced depression of cardiac myocyte contractility. Am J Physiol Regul Integr Comp Physiol 276: R265–R276, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev 7: 548–562, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Lomas-Niera JL, Perl M, Chung CS, Ayala A. Shock and hemorrhage: an overview of animal models. Shock 24, Suppl 1: 33–39, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Magnotti LJ, Upperman JS, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg 228: 518–527, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function: ten years after, and continuing. Circ Res 93: 388–398, 2003 [DOI] [PubMed] [Google Scholar]
- 21. McDonough KH, Giaimo M, Quinn M, Miller H. Intrinsic myocardial function in hemorrhagic shock. Shock 11: 205–210, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Mehra VC, Ramgolam VS, Bender JR. Cytokines and cardiovascular disease. J Leukoc Biol 78: 805–818, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Murphy AM, Kogler H, Georgakopoulos D, McDonough JL, Kass DA, Van Eyk JE, Marban E. Transgenic mouse model of stunned myocardium. Science 287: 488–491, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Parrillo JE, Burch C, Shelhamer JH, Parker MM, Natanson C, Schuette W. A circulating myocardial depressant substance in humans with septic shock. Septic shock patients with a reduced ejection fraction have a circulating factor that depresses in vitro myocardial cell performance. J Clin Invest 76: 1539–1553, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Port JD, Bristow MR. Altered beta-adrenergic receptor gene regulation and signaling in chronic heart failure. J Mol Cell Cardiol 33: 887–905, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Sambol JT, Lee MA, Caputo FJ, Kawai K, Badami C, Kawai T, Deitch EA, Yatani A. Mesenteric lymph duct ligation prevents trauma/hemorrhage shock-induced cardiac contractile dysfunction. J Appl Physiol 106: 57–65, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sayeed MM. Signaling mechanisms of altered cellular responses in trauma, burn, and sepsis: role of Ca2+. Arch Surg 135: 1432–1442, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Shahani R, Klein LV, Marshall JG, Nicholson S, Rubin BB, Walker PM, Lindsay TF. Hemorrhage-induced alpha-adrenergic signaling results in myocardial TNF-α expression and contractile dysfunction. Am J Physiol Heart Circ Physiol 281: H84–H92, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Sutherland FJ, Hearse DJ. The isolated blood and perfusion fluid perfused heart. Pharmacol Res 41: 613–627, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Trafford AW, Diaz ME, O'Neill SC, Eisner DA. Integrative analysis of calcium signalling in cardiac muscle. Front Biosci 7: d843–852, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Yang S, Hu S, Hsieh YC, Choudhry MA, Rue LW, 3rd, Bland KI, Chaudry IH. Mechanism of IL-6-mediated cardiac dysfunction following trauma-hemorrhage. J Mol Cell Cardiol 40: 570–579, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Yatani A, Shen YT, Yan L, Chen W, Kim SJ, Sano K, Irie K, Vatner SF, Vatner DE. Down regulation of the L-type Ca(2+) channel, GRK2, and phosphorylated phospholamban: protective mechanisms for the denervated failing heart. J Mol Cell Cardiol 40: 619–628, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Yatani A, Xu DZ, Irie K, Sano K, Jidarian A, Vatner SF, Deitch EA. Dual effects of mesenteric lymph isolated from rats with burn injury on contractile function in rat ventricular myocytes. Am J Physiol Heart Circ Physiol 290: H778–H785, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Yatani A, Xu DZ, Kim SJ, Vatner SF, Deitch EA. Mesenteric lymph from rats with thermal injury prolongs the action potential and increases Ca2+ transient in rat ventricular myocytes. Shock 20: 458–464, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Yokoyama T, Vaca L, Rossen RD, Durante W, Hazarika P, Mann DL. Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart. J Clin Invest 92: 2303–2312, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yu HP, Shimizu T, Choudhry MA, Hsieh YC, Suzuki T, Bland KI, Chaudry IH. Mechanism of cardioprotection following trauma-hemorrhagic shock by a selective estrogen receptor-beta agonist: up-regulation of cardiac heat shock factor-1 and heat shock proteins. J Mol Cell Cardiol 40: 185–194, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Zallen G, Moore EE, Johnson JL, Tamura DY, Ciesla DJ, Silliman CC. Posthemorrhagic shock mesenteric lymph primes circulating neutrophils and provokes lung injury. J Surg Res 83: 83–88, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Ziolo MT, Maier LS, Piacentino V, 3rd, Bossuyt J, Houser SR, Bers DM. Myocyte nitric oxide synthase 2 contributes to blunted beta-adrenergic response in failing human hearts by decreasing Ca2+ transients. Circulation 109: 1886–1891, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Zuckerbraun BS. Estrogen therapy for trauma/hemorrhage: the heart follows suit. Crit Care Med 37: 2471–2473, 2009 [DOI] [PubMed] [Google Scholar]