Figure 2. PKCδ inhibition blunts FAK activity and diminished cytoskeletal stiffness.
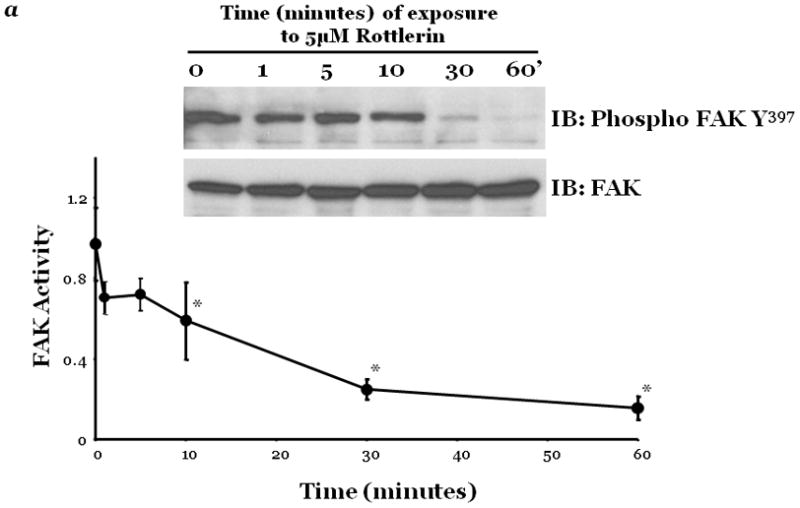
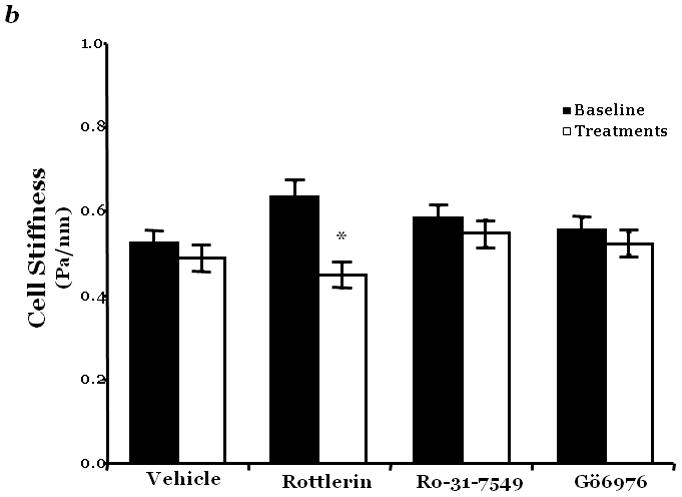
Panel a, FAK activity was determined by measuring the level of phosphorylation at FAK Y397 by immunoblot analysis at indicated times following incubation of endothelial cells derived from the epididymal fat pad (FPEC) with 5μM rottlerin. The immunoblotted membranes were subsequently stripped and reprobed for FAK. Immunoblot signals were quantitated by densitometry and the level of FAK activity is presented as the mean±SE of the ratio of FAK Y397 phosphorylation to total FAK. Panel b, barrier function is dictated by changes in both contractile and adhesive forces, thus to measure changes in the contractile forces, cytoskeletal stiffness was assessed in lung microvascular endothelial cells (LMVEC) which were overlaid with ferrimagnetic beads, coated with the integrin receptor-specific peptide sequence (Arg-Gly-Asp; RGD), forming apical focal adhesions between the LMVEC and the ferrimagnetic beads. The beads were then twisted, using a magnet, and the resistant force was measure both before treatment (i.e., baseline) and in the same cultures 30 minutes following exposure to vehicle, 250nM Ro-31-7549 (a chemical inhibitor with specificity for PKCα, β, γ, ε), 10nM Gö6976 (a chemical inhibitor with specificity for PKCα, β, γ), or 5μM rottlerin. Data are presented as mean±SE (n=280–520 cells). *p<0.05 vs. vehicle with respective treatment. Panel a: Reprinted from Harrington, E.O., et al., 2005. PKCδ regulates endothelial basal barrier function through modulation of RhoA GTPase activity. Experimental Cell Research, 308:407–421. Panel b: Reprinted from Klinger, J.R., et al., 2007. Rottlerin causes pulmonary edema in vivo: A possible role for PKCδ. Journal of Applied Physiology, 103:2084–2094.
