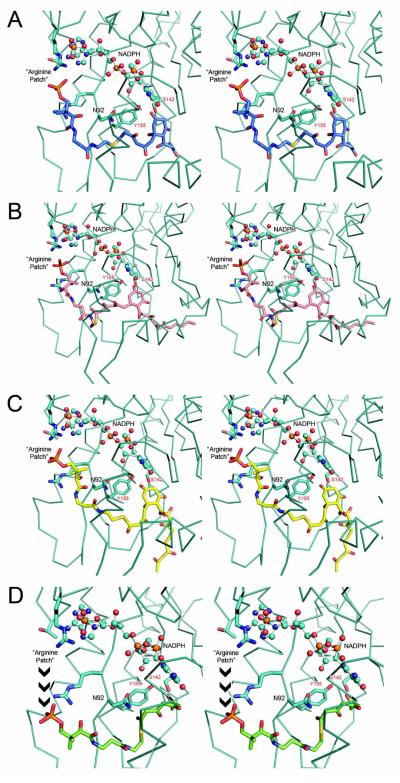
Figure 8.
Stereo views of various PPT-linked polyketides docked to hedKR, with monomer A portrayed in teal in each panel. (A) C7-C12 cyclized, 16-carbon polyketide (blue); (B) the putative C7-C12 cyclized, 24-carbon hedKR polyketide substrate (pink); and (C) C5-C10 cyclized, 22-carbon polyketide (yellow). These in silico docking analyses demonstrate that hedKR can accommodate the polyketide substrates portrayed in (A)-(C). (D) None of the 8-carbon polyketide (green) docking solutions placed the PPT phosphate within the “arginine patch,” which is the proposed docking site for ACP. These results suggest that the reduced tetraketide compound (13) is formed after release of the thioester-bound polyketide from holo-ACP, spontaneous pyrone ring cyclization, and C7-reduction of the molecule by hedKR.