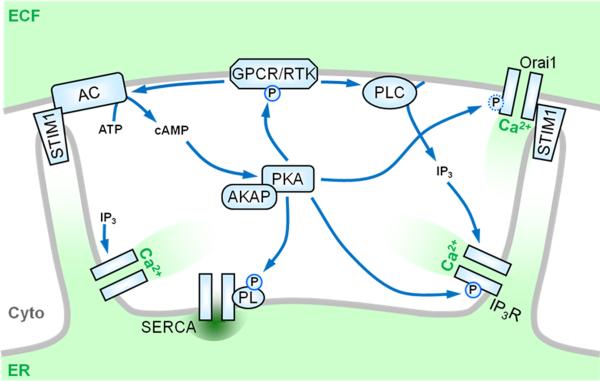
Figure 2.
Connection between store-operated Ca2+ entry (SOCE) and PKA. SOCE, and its principal mediators STIM1 and Orai1, play increasingly important roles in the migration of a number of cell types. During SOCE, signals generated by receptors (e.g. G-protein coupled receptors (GPCR) or receptor tyrosine kinases (RTK)) at the plasma membrane activate phospholipases C (PLC) to generate IP3, which causes the release of Ca2+- from intracellular stores via the IP3R. Store depletion activates STIM1, which re-localizes within the ER membrane to allow interaction with and activation of the plasma membrane Ca2+ channel Orai1. Store-depletion has also been shown to stimulate adenylyl cyclase (AC) and activate PKA. Reciprocally, PKA may regulate the SOCE pathway at a number of levels, e.g. through phosphorylation and modulation of many PLC-coupled receptors, the IP3R, phospholamban associated with the sarco-/endoplasmic reticulum Ca2+-ATPase (SERCA), and possibly Orai1 itself (see Figure 3).