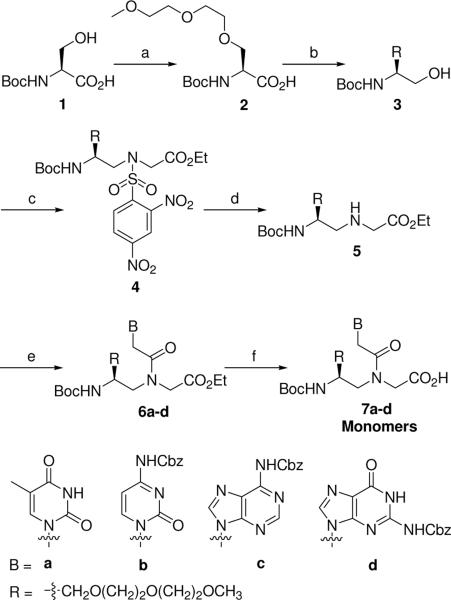
Scheme 1.
Synthesis of R-MPγPNA Monomers Reagents and conditions: (a) NaH, BrCH2CH2OCH2CH2OCH3, DMF, 0 °C, 68%; (b) isobutyl chloroformate, NaBH4, NMM, DME, 0 °C→rt, 84%; (c) DIAD, 2,4-dinitrobenzenesulfonyl glycine ethyl ester, TPP, THF, 0 °C→rt, 56%; (d) n-propylamine, CH2Cl2, rt, 81%; (e) carboxymethylene nucleobase, DCC, DhObtOH, DMF, 50 °C, 67–85%; (f) 2M NaOH/THF (1:1), 0 °C, 85–98%.