Abstract
AIMS
An integrated population pharmacokinetic–pharmacodynamic model was developed with the following aims: to simultaneously describe pharmacokinetic behaviour of sugammadex and rocuronium; to establish the pharmacokinetic–pharmacodynamic model for rocuronium-induced neuromuscular blockade and reversal by sugammadex; to evaluate covariate effects; and to explore, by simulation, typical covariate effects on reversal time.
METHODS
Data (n = 446) from eight sugammadex clinical studies covering men, women, non-Asians, Asians, paediatrics, adults and the elderly, with various degrees of renal impairment, were used. Modelling and simulation techniques based on physiological principles were applied to capture rocuronium and sugammadex pharmacokinetics and pharmacodynamics and to identify and quantify covariate effects.
RESULTS
Sugammadex pharmacokinetics were affected by renal function, bodyweight and race, and rocuronium pharmacokinetics were affected by age, renal function and race. Sevoflurane potentiated rocuronium-induced neuromuscular blockade. Posterior predictive checks and bootstrapping illustrated the accuracy and robustness of the model. External validation showed concordance between observed and predicted reversal times, but interindividual variability in reversal time was pronounced. Simulated reversal times in typical adults were 0.8, 1.5 and 1.4 min upon reversal with sugammadex 16 mg kg−1 3 min after rocuronium, sugammadex 4 mg kg−1 during deep neuromuscular blockade and sugammadex 2 mg kg−1 during moderate blockade, respectively. Simulations indicated that reversal times were faster in paediatric patients and slightly slower in elderly patients compared with adults. Renal function did not affect reversal time.
CONCLUSIONS
Simulations of the therapeutic dosing regimens demonstrated limited impact of age, renal function and sevoflurane use, as predicted reversal time in typical subjects was always <2 min.
Keywords: population pharmacokinetics and pharmacodynamics, nonlinear mixed effects model, paediatric, elderly, renal function
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Pharmacokinetics and pharmacodynamics of rocuronium are well known.
A mechanistical pharmacokinetic–pharmacodynamic model based on limited data describing rocuronium and sugammadex pharmacokinetics has been published.
However, the available pharmacokinetic–pharmacodynamic model for the effects of sugammadex on rocuronium-induced neuromuscular blockade has limited predictive power.
WHAT THIS STUDY ADDS
A pharmacokinetic–pharmacodynamic model is described for rocuronium and sugammadex, based on physiological principles and on data covering a wide range of ages and renal function. From this model, more accurate predictions can be made about sugammadex-mediated reversal of rocuronium-induced neuromuscular blockade, in unexplored regimens or populations.
Introduction
Neuromuscular blocking agents (NMBAs) are frequently used during anaesthesia to facilitate tracheal intubation, artificial ventilation and surgical procedures. Reversal agents (e.g. the anticholinesterases neostigmine or edrophonium) are often administered to accelerate recovery from neuromuscular blockade (NMB) and prevent postoperative residual NMB [1]. The use of anticholinesterases to reverse residual neuromuscular block is efficacious only if recovery is already established. Even in these circumstances, the full effect of anticholinesterases takes up to 10 min to achieve [2].
Sugammadex is a modified γ-cyclodextrin and the first selective relaxant binding agent designed to encapsulate the aminosteroidal NMBA, rocuronium. Sugammadex forms a 1:1 host–guest inclusion complex with rocuronium in plasma [3]. Free rocuronium molecules in plasma are captured by sugammadex, resulting in rapid decrease of the free rocuronium plasma concentration. This creates a concentration gradient between free rocuronium in the effect compartment (the neuromuscular junction) and plasma. As a result, free rocuronium molecules return to the plasma, where they are captured by sugammadex, leading to rapid reversal of NMB. Typical reductions in time to reversal of a moderate level of NMB range from between 30 and 60 min after spontaneous reversal to approximately 2 min after sugammadex.
The efficacy and safety of sugammadex have been established in various clinical trials [4–17]. Treatment options for sugammadex include rapid reversal at 3 min after rocuronium using 16 mg kg−1 sugammadex, deep blockade reversal using 4 mg kg−1 sugammadex administered at recovery to one or two post-tetanic counts or 15 min post 0.6 mg kg−1 rocuronium and moderate blockade reversal using 2 mg kg−1 at reappearance of the second twitch (T2) of the train-of-four (TOF) monitoring.
The binding of sugammadex and rocuronium itself is unaffected by covariate effects, but the rate of complexation is dependent on the mutual availability of the two compounds. The likelihood to interact is dependent on the pharmacokinetics of the compounds, which can be affected by covariates.
Sugammadex distributes in the extracellular water of the body and binds negligibly to plasma proteins and erythrocytes. Metabolism of sugammadex is very limited; thus, the compound is primarily eliminated via renal excretion of the unchanged product [18]. The rate of clearance of sugammadex is similar to the glomerular filtration rate. Observed age effects can be accounted for by the decreasing renal function with age [11]. On a clinical trial level, no major effects of gender or race (Japanese vs. Caucasians) have been observed. Within the dose range studied (0.1–96 mg kg−1) sugammadex exhibits dose-proportional pharmacokinetics (PK) [19]. When rocuronium is encapsulated by sugammadex, it behaves pharmacokinetically like sugammadex [3].
On a mechanistic level, the PK interaction of free rocuronium, free sugammadex and the complex, and their subsequent effects on NMB have been described [20]. A thorough population pharmacokinetic–pharmacodynamic (PK–PD) analysis has not been performed. Effects of the covariates bodyweight, age, gender, race and renal function on sugammadex PK and subsequently on sugammadex-mediated reversal of NMB have not yet been studied in great detail. Effects of sevoflurane anaesthesia on rocuronium have been established [21–24], but the impact on sugammadex-mediated reversal of NMB has not been investigated in a population PK–PD analysis.
Subsequent aims in model development were as follows: to establish the PK–PD model for rocuronium-induced NMB and its reversal by sugammadex; to evaluate potential covariate effects in a wide range of patient demographic characteristics, such as like age, bodyweight, gender, race and creatinine clearance, and the effect of sevoflurane anaesthesia on reversing rocuronium-induced NMB by sugammadex; and finally, to explore, by simulation, typical covariate effect combinations on the reversal time.
Methods
Clinical trials
Data from 10 clinical trials were used [4–14]; eight clinical trials were used for model building, while two clinical trials were used for external validation. These studies investigated the use of sugammadex in reversing rocuronium-induced NMB and the safety, tolerability and PK of rocuronium and sugammadex. Each study was conducted in accordance with principles of Good Clinical Practice and was approved by the appropriate institutional review boards and regulatory agencies. An overview of the design of the clinical trials, including patient population, rocuronium and sugammadex treatment, type of anaesthesia and PK assessments for both compounds can be found in Table 1.
Table 1.
Clinical trials included in model building and external validation
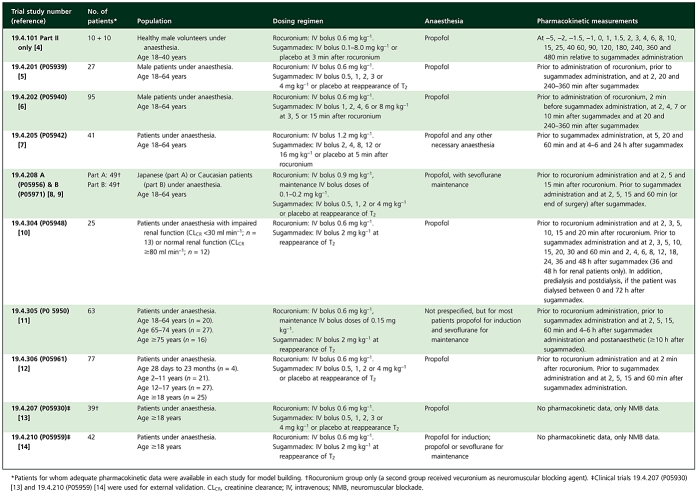 |
Rocuronium and sugammadex plasma concentrations
Plasma concentrations of rocuronium and sugammadex were measured at the Department of Bioanalysis, MSD, Oss, The Netherlands, using two separate, validated liquid chromatographic assay methods with mass spectrometric detection [5, 25]. The assays were conducted in full compliance with Good Laboratory Practice regulations. As the assay methods used to determine the plasma concentrations of sugammadex and rocuronium did not discriminate between complexed and noncomplexed sugammadex and rocuronium, the plasma concentrations reported relate to total plasma concentrations. The lower limit of quantification was 2 ng ml−1 for rocuronium and 0.1 µg ml−1 for sugammadex. The intra- and interassay coefficients of variation were within 2.5–11.2 and 4.1–14.5%, respectively, for rocuronium [5] and were within 3.4–5.6 and 4.9–7.3%, respectively, for sugammadex [25].
Assessment of NMB, T2 twitch height and T4/T1 twitch ratio
Neuromuscular blockade data were collected by means of acceleromyography using the TOF-Watch® SX (Organon Ireland Ltd, a Division of Merck and Co., Inc., Swords, County Dublin, Ireland) according to the guidelines for Good Clinical Research Practice in pharmacodynamic (PD) studies [26]. Recently, the use of acceleromyography to establish the potency of NMBAs and reversal agents was shown to be justified [27]. In this technique, a TOF electrical stimulus of the ulnar nerve is applied, delivering four successive monophasic pulses every 15 s. Successive twitches of the TOF are termed T1, T2, T3 and T4, respectively. The efficacy for sugammadex-mediated reversal of NMB was determined by means of the reversal time, defined as the time from the start of administration of sugammadex/placebo to recovery to a T4/T1 twitch ratio of 0.7 (TOF70), 0.8 and 0.9 (TOF90), the latter being the primary clinical end-point of the trials.
Data dilution
Data dilution was applied to reduce the amount of TOF data, being on average >500 assessments per patient. Data points were removed without reducing information content for the PK–PD model and retaining a balanced distribution of data points, which matched timewise the clinically relevant concentration range. The following algorithm was applied: all assessments within 1 min before and up to 3 min after the first rocuronium dose; no assessments between 3 min after first rocuronium dose up to 1 min before sugammadex dose or placebo; all assessments within 1 min before and up to 3 min after the sugammadex dose or placebo; and the number of assessments selected in the recovery part of the profile was determined by the steepness of the curve (elapsed time between reappearance of the T4/T1 twitch ratio and time of TOF70).
Patient characteristics
The data set used for modelling of the PK interaction model consisted of 426 patients and 20 healthy volunteers. From this data set, 29 patients were excluded from the PK–PD modelling. Reasons for exclusion, according to an independently acting adjudication committee, were as follows: TOF data not available; protocol violations affecting assessment of NMB; and unreliable TOF profile, probably due to movement of the patient. The PK–PD model linking rocuronium exposure to NMB was developed on a subset of 59 patients who were treated with rocuronium alone, i.e. they received placebo instead of sugammadex (placebo patients). For the development of the PK–PD model describing rocuronium and sugammadex exposure and their effects on NMB, a subset of 338 patients treated with rocuronium and sugammadex were included. Patient characteristics for the data sets are presented in Table 2.
Table 2.
Demographics and distribution of patients among age classes, renal function, gender, race and sevoflurane use
| Covariate | Data set | n | Distribution of patients by class for each data set, n | Mean | SD | Minimum | Median | Maximum |
|---|---|---|---|---|---|---|---|---|
| Bodyweight (kg) | Pharmacokinetic model | 446 | NA | 72.5 | 19.2 | 9.60 | 74.5 | 139 |
| NMB with rocuronium | 59 | NA | 69.6 | 20.8 | 11.2 | 74.5 | 107 | |
| NMB with rocuronium + sugammadex reversal | 338 | NA | 73.0 | 19.3 | 9.60 | 75.0 | 139 | |
| Age (years) | Pharmacokinetic model | 446 | Infant/child/adolescent/elderly/old elderly*: 4/21/27/342/34/18 | 42.4 | 18.7 | 1 | 43 | 91 |
| NMB with rocuronium | 59 | Infant/child/adolescent/elderly/old elderly*: 1/4/6/48/0/0 | 35.4 | 16.8 | 1 | 36 | 64 | |
| NMB with rocuronium + sugammadex reversal | 338 | Infant/child/adolescent/elderly/old elderly*: 3/17/21/247/33/17 | 43.4 | 19.5 | 1 | 44 | 91 | |
| CLCR (ml min−1) | Pharmacokinetic model | 446 | Renal function, healthy/renally impaired†: 432/14 | 118 | 40.8 | 4.30 | 119 | 239 |
| NMB with rocuronium | 59 | Renal function, healthy/renally impaired†: 59/0 | 134 | 40.3 | 53.3 | 124 | 239 | |
| NMB with rocuronium + sugammadex reversal | 338 | Renal function, healthy/renally impaired†: 324/14 | 115 | 41.5 | 4.30 | 117 | 221 | |
| Gender | Pharmacokinetic model | 446 | Male/female: 289/157 | NA | NA | NA | NA | NA |
| NMB with rocuronium | 59 | Male/female: 43/16 | NA | NA | NA | NA | NA | |
| NMB with rocuronium + sugammadex reversal | 338 | Male/female: 212/126 | NA | NA | NA | NA | NA | |
| Race | Pharmacokinetic model | 446 | Non-Asian/Asian: 393‡/53 | NA | NA | NA | NA | NA |
| NMB with rocuronium | 59 | Non-Asian/Asian: 52/7 | NA | NA | NA | NA | NA | |
| NMB with rocuronium + sugammadex reversal | 338 | Non-Asian/Asian: 304§/34 | NA | NA | NA | NA | NA | |
| Sevoflurane use¶ | Pharmacokinetic model | NA | No/yes: NA | NA | NA | NA | NA | NA |
| NMB with rocuronium | 59 | No/yes: 45/14 | NA | NA | NA | NA | NA | |
| NMB with rocuronium + sugammadex reversal | 338 | No/yes: 235/103 | NA | NA | NA | N/A | NA |
Infant, 28 days to 23 months (but all were aged 1 year); child, 2–11 years; adolescent, 12–17 years; adult, 18–64 years; elderly, 65–74 years; and old elderly, ≥75 years.
Renal impairment defined as having a CLCR <30 ml min−1 (only applicable for the adult and elderly population; there were no renally impaired patients among the paediatric population).
Including 383 Caucasian, nine Afro-American and one Hispanic subject.
Including 295 Caucasian and nine Afro-American subjects.
Sevoflurane was included as a dichotomous covariate, being zero when no sevoflurane was administered.
Creatinine clearance (CLCR) for adult subjects (≥18 years) was calculated according to Cockcroft–Gault [37]. For paediatric subjects (<18 years), CLCR was based upon the formulae of Schwartz [38]. To obtain the uncorrected CLCR in paediatric subjects, the body surface area as calculated with the formula of Dubois and Dubois [39] was used. Age-matched height values taken from growth curves [44] were used for imputation of missing height values in paediatric patients. In case of a missing serum creatinine value, the formula: CLCR (ml min−1) = 183–1.34 × age (years) was used to impute CLCR for a particular adult subject; the formula: CLCR (ml min−1) = 53.8 + age1.75 (years) was used for missing serum CLCR in the paediatric population. These relationships were established upon fitting of age and CLCR values in the data set. There were no missing values for bodyweight and age. NA, not applicable; NMB, neuromuscular blockade.
Pharmacokinetic–pharmacodynamic analysis
The population PK and PK–PD analyses were performed using the nonlinear mixed effects modelling approach.
The software package NONMEM (version VI, level 1.0; ICON Development Solutions, Ellicott City, MD, USA) was used in the analysis. SPlus (S-PLUS 6.2 Professional Edition; TIBCO Software Inc., Palo Alto, CA, USA) and R (v2.5.0; R Development Core Team 2010, R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org) were used for postprocessing of NONMEM output. Perl (v5.8.8; J. Hietaniemi, Comprehensive Perl Archive Network, http://www.cpan.org, 26 March 2003.) and Perl-speaks NONMEM (PsN v2.2.4; Perl-speaks-NONMEM is copyright ©2008 by Mats Karlsson, Niclas Jonsson and Andrew Hooker. Department of Pharmaceutical Biosciences, Division of Pharmacokinetics and Drug Therapy, Uppsala University, Uppsala, Sweden) were used to perform NONMEM jobs, automatic covariate model-building and model evaluation (bootstrap and log-likelihood profiling) [28].
Pharmacokinetic parameter estimates for rocuronium and sugammadex were obtained by simultaneous fitting of the concentration–time data. Estimates of the rocuronium PD parameters were obtained by fitting NMB profiles of placebo patients in combination with post hoc PK parameter estimates. For active sugammadex-treated patients, individual estimates for rocuronium PD parameters were obtained in a post hoc step, applying the rocuronium model to NMB profiles up to the time of sugammadex administration. Subsequently, the post hoc PK parameters and post hoc rocuronium PD parameters were used to estimate sugammadex PD parameters. Modelling strategies with simultaneous estimation of the rocuronium PD and sugammadex effects were not successful. Sugammadex effects were optimized at the expense of the rocuronium parameters.
During model development, first-order conditional estimation with additive error models for residual variability was used, and first-order conditional with interaction in case of proportional and additive plus proportional models for residual variability [29, 30]. The final model was derived using first-order conditional estimation.
Pharmacokinetic model development
The previously published model [20] describing the PK of free rocuronium, free sugammadex and the complex applying three-compartmental models for each, was used as starting point for model development. Pharmacokinetic parameters for the complex were set equal to free sugammadex PK parameters. Complexation occurred in the central compartments; the model did not take into account complexation in the peripheral compartments. Complexation is much faster compared with the distributional process, thereupon rocuronium and sugammadex are unlikely to have similar tissue distributions. Complex formation was described by dynamic interaction, with rate constants for association (k1) and dissociation (k2), where complex formation occurs non-instantaneously. The in vitro assessed dissociation constant (kd) was included as a fixed parameter (0.0559 µm). During model building, the equilibrium complex formation model [20], where complexation occurs instantaneously, was also evaluated.
Pharmacokinetic–pharmacodynamic model development
An effect compartment linked to the central rocuronium compartment was used to fit the dissociation (keo) between plasma concentrations and NMB. The following sigmoidal Emax model was applied to correlate effect compartment concentrations (Ceff) with NMB:
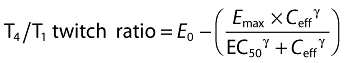 |
where E0 is the baseline T4/T1 twitch ratio; Emax (set equal to E0 for each individual) is maximal reduction in neuromuscular function corresponding to full muscle relaxation, i.e. T4/T1 twitch ratio of 0; EC50 is the rocuronium concentration in the effect compartment required to produce a 50% reduction in neuromuscular function; and γ is the Hill coefficient. This model framework is well accepted for the fitting of NMB [23, 24, 31, 32].
The fast onset of sugammadex on NMB was modelled by modulation of the output rate of rocuronium from the effect compartment. The effect of sugammadex was incorporated in the rocuronium PD model as an additional route of elimination of rocuronium from the effect compartment.
The rate of elimination by this route is dependent on rate constant (ks) and the free sugammadex concentration in the central sugammadex compartment. This mathematical model combined with the effect compartmental model mimics the underlying physiological process as described by Bom et al. [3]. After intravenous injection, sugammadex distributes rapidly over the extracellular volume. Sugammadex does not enter cells owing to the large size of the γ-cyclodextrin skeleton and the eight negatively charged end-groups on the side-chains. It is not clear whether sugammadex can enter the neuromuscular junction, but the high metabolic demand in the neuromuscular junction (nonstop synthesis of acetylcholine, no re-uptake) indicates that the target site is well perfused. A rapid reduction in the free rocuronium concentration in the tissue surrounding the neuromuscular junction creates a concentration gradient, which forces rocuronium molecules to move from the neuromuscular junction towards the surrounding tissue, where they can be encapsulated.
An alternative model applying complex formation in the biophase was judged not feasible because rate constants for complex formation cannot be distinguished from distributional processes in tissue. In addition, it is debatable whether tissue distribution is similar for rocuronium and sugammadex.
Size effects
Bodyweight-based allometric scaling [33–36] was applied to the PK parameters for clearances and distribution volumes and the rate constants linking PK to NMB. The allometric coefficients were fixed at 0.75, 1 and −0.25, respectively. For confirmation of the validity of this approach, the parameters were screened for residual bodyweight effects in the covariate selection procedure.
Renal function
Creatinine clearance for adults and the elderly was derived from creatinine concentration, age and bodyweight using the formulae of Cockcroft–Gault [37]. In the paediatric age range, creatinine clearance was derived using the formulae of Schwartz et al. [38] followed by denormalization by body surface area [39].
Random effects
Exponential error models were utilized for interindividual variability (IIV) in PK and PD parameters, except for E0 where the proportional error model was investigated. Covariance between the PK parameters and between the PD parameters was also investigated.
The log-additive residual error model was applied for concentration data. An additive residual error model was used for the T4/T1 twitch ratio and T2 twitch height data. Other more complex error models were also evaluated.
Covariate analysis
The demographic factors bodyweight, age, gender, race (Asian and non-Asian) and creatinine clearance were considered for inclusion in the model describing rocuronium and sugammadex PK parameters. Effects on complex formation (kd = k2/k1) were not evaluated; although changes in blood composition could potentially affect the rate of complexation, it was believed that this should not be related to the investigated covariates.
Sugammadex is predominantly cleared by the renal route; therefore, creatinine clearance was related a priori to clearance of sugammadex at the level of the structural model and was not subject to covariate screening. As size effects were already included in creatinine clearance, clearance of sugammadex was not allometrically scaled; it was assumed that creatinine clearance was a better predictor for maturation and degeneration effects than scaling by size with the allometric approach. The shape of the relation was empirically determined according to the following equation:
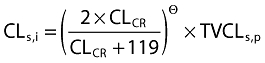 |
where CLs,i and TVCLs,p represent individual and typical sugammadex clearance, respectively, CLCR represents creatinine clearance, and Θ determines the shape of the relationship.
Sevoflurane use was tested for effects on the rocuronium PD parameters keo and EC50[23, 24] and the sugammadex PD parameter ks. Sevoflurane use was included as a dichotomous covariate. Typically, patients received sevoflurane anaesthesia prior to rocuronium administration until reversal of NMB. Therefore, other models, such as an on–off model reflecting sevoflurane administration in time, or even more sophisticated models using sevoflurane concentrations in time, were not feasible.
Model selection criteria
Covariate modelling was performed by applying the automated stepwise evaluation of covariates as implemented in PsN. For continuous covariates, first the linear relationship was tested and, when included, the exponential model was evaluated on further improvement of the relationship. Categorical covariates were included, with an extra parameter added for each except the most common category.
The statistical significance for inclusion of a covariate relation or an IIV term in the model was assessed by comparison of the objective function before and after the inclusion [forward inclusion objective function value (OFV) 7.88, P = 0.005, backward exclusion OFV 10.8 P = 0.001]. Furthermore, covariates were retained in the model when the effect differed significantly from zero (95% confidence interval did not include zero) and the condition number was <1000. Inclusion of covariate effects should be accompanied by a decrease in unexplained variability to be deemed clinically meaningful.
Internal validation
During PK model development, goodness-of-fit plots were used to assess the performance of the subsequent models. Given the heterogeneous nature of the data, dosing regimens for the larger part varying on an individual basis and the wide range of covariate values, model performance for the PK–PD model could not be assessed with a simple visual predictive check (VPC)-based analysis. Instead of inspection of the goodness of fit at the data-point level, the clinical end-point reversal time was used to assess true model performance. Posterior predictive checking on the clinical end-point (reversal time, defined as the time to attainment of T4/T1 twitch ratios of both 0.9 and 0.7) was applied to investigate performance and goodness of fit of the PK–PD model.
Shrinkage values were calculated, and bootstrapping (n = 1000) was applied to the final model to demonstrate the robustness of the PK and PD parameter estimates.
External validation
The final PK–PD interaction model was externally validated by applying it to the PD data from clinical trials 19.4.207 (P05930) [13] and 19.4.210 (P05959) [14] (Table 1). Model performance was validated through posterior predictive checking by comparing model-based predicted reversal time to TOF90 and TOF70 for scenarios that were investigated in these trials. The PK and PD parameters and their associated IIV were simulated by resampling 100 times from the interindividual distribution of the model parameters. Median patient demographic data for each treatment group was used to account for covariate effects. Individual covariates were not taken into account because these effects were small compared with the high level of IIV. Residual error terms were set to zero. Sampling frequency in the simulations was one sample every 15 s, in line with sampling frequency of the TOF-Watch. As a result, individual reversal times were discrete values and a multiple of 0.25 min.
Simulations
Simulations were carried out for three scenarios designating immediate reversal, deep blockade reversal and moderate blockade reversal. Simulations were performed for typical subjects (Table 3) to predict the effect of age and renal function on reversal time. In addition, the effect of sevoflurane on reversal time was evaluated in typical subjects (aged 40 years, weight 75 kg, creatinine clearance 100 ml min−1). Simulations were based on population parameter estimates, and IIV was taken into account; model parameters were resampled 100 times from the interindividual distribution. Residual error terms were set to zero.
Table 3.
Typical subjects used in simulations
| Population | Infant 1 year | Child 7 years | Adolescent 15 years | Adult 40 years | Elderly 75 years |
|---|---|---|---|---|---|
| Bodyweight (kg)* | 10.3 | 23.0 | 56.2 | 75 | 75 |
| Height (cm)* | 75.5 | 122 | 170 | NA | NA |
| Stages of renal function†‡ | |||||
| Normal renal function | 25.7 | 51.2 | 95.3 | 100 | 80 |
| Mild renal impairment | 12.9 | 25.6 | 47.6 | 50 | 50 |
| Moderate renal impairment | 7.72 | 15.3 | 28.6 | 30 | 30 |
| Severe renal impairment | 2.57 | 5.12 | 9.53 | 10 | 10 |
Age-matched bodyweight and height values for typical paediatric subjects were taken from growth curves [44].
Paediatric creatinine clearance (CLCR) values were scaled on body surface area (BSA) as calculated by Dubois and Dubois [39], as follows: CLCR = 100 × (BSApaed/BSAadult).
CLCR (ml min−1) limits were used to define stages of renal function.
Results
The final PK–PD interaction model together with an example of the response profiles for free and complexed rocuronium and sugammadex plasma concentrations, free rocuronium concentration in the effect compartment and the accompanying T4/T1 twitch ratio upon moderate block reversal is given in Figure 1. In Table 4, the estimates of the PK and PD parameters, including covariate relations, are given together with shrinkage values and confidence intervals obtained from the bootstrap analysis.
Figure 1.
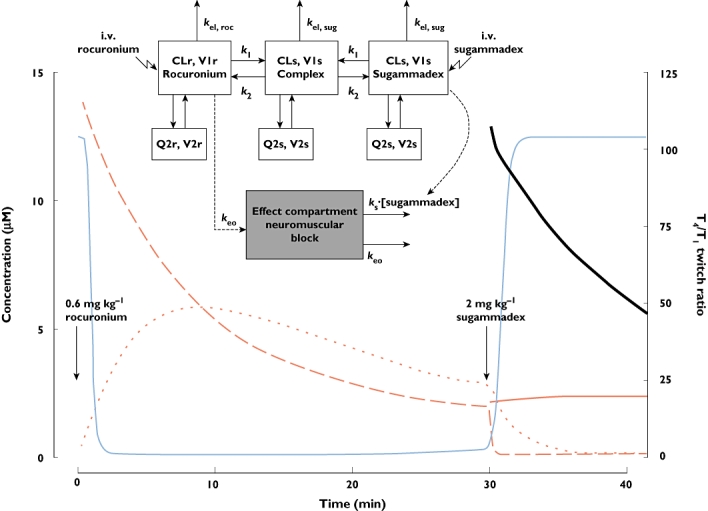
The final pharmacokinetic–pharmacodynamic interaction model and illustration of pharmacokinetic and pharmacodynamic response profiles. Abbreviations: CLr, rocuronium clearance; CLs, sugammadex clearance; k1, rate constant of association between sugammadex and rocuronium; k2, rate constant of dissociation; kel,roc, rate of elimination of rocuronium; kel,sug, rate of elimination of sugammadex; keo, distribution rate constant between central and effect compartments; ks, rate constant; Q2r, intercompartment clearance of rocuronium from the central to the peripheral compartment; Q2s, intercompartment clearance of sugammadex from the central to the peripheral compartment; V1r, volume of distribution of rocuronium in the central compartment; V1s, volume of distribution of sugammadex in the central compartment; V2r, volume of distribution of rocuronium in the effect compartment; and V2s, volume of distribution of sugammadex in the effect compartment. Rocuronium plasma, free ( ); Rocuronium plasma, free + complex (
); Rocuronium plasma, free + complex ( ); Rocuronium effect compartment, free (
); Rocuronium effect compartment, free ( ); sugammadex plasma, free + complex (
); sugammadex plasma, free + complex ( ); T4/T1 twitch ratio (
); T4/T1 twitch ratio ( )
)
Table 4.
Parameter estimates and covariate relations of the final pharmacokinetic–pharmacodynamic interaction model
| Parameterization | Unit | Population estimate | RSE (%) | Bootstrap (n = 1000) 95% CI |
|---|---|---|---|---|
| Pharmacokinetic model rocuronium | ||||
| CL = CLAGE×Θ× (BW/70)0.75 | l min−1 | 0.269 | 1.79 | 0.259–0.277 |
| CLAGE = 1 +Θ× (AGE – 43.0) | −0.00678 | 16.1 | −0.00887 to −0.00490 | |
| IIV CL (shrinkage 12%) | % | 32.4 | 11.1 | 29.0–35.6 |
| V1 = V1CR×Θ× (BW/70)1 | l | 4.73 | 2.68 | 4.46–4.98 |
| V1CR = Exp[Θ× (CR – 119)] | −0.00143 | 26.4 | −0.00214 to −0.00073 | |
| IIV V1 (shrinkage 28%) | % | 23.8 | 33.6 | 17.6–31.1 |
| Q2 = Q2RAC×Θ× (BW/70)0.75 | l min−1 | 0.279 | 5.27 | 0.253–0.309 |
| Non-Asian: Q2RAC = 1 | ||||
| Asian: Q2RAC = 1 +Θ | −0.212 | 38.2 | −0.340 to −0.060 | |
| V2 = V2AGE×Θ× (BW/70)1 | l | 6.76 | 2.22 | 6.48–7.12 |
| V2AGE = Exp[Θ× (AGE – 43.0)] | 0.00613 | 20.6 | 0.00373–0.00851 | |
| IIV V2 (shrinkage 37%) | % | 32.2 | 21.6 | 28.5–35.9 |
| Residual error (shrinkage 14%) | % | 20.0 | 10.8 | 17.9–22.1 |
| Pharmacokinetic model sugammadex | ||||
| CL = CLBW× (REN ×Θ) | l min−1 | 0.093 | 1.47 | 0.0902–0.0958 |
| REN = [2 × CR/(CR+119)]Θ | 1.29 | 4.77 | 1.19–1.39 | |
| CLBW = 1 +Θ× (BW – 74.5) | 0.00378 | 19.1 | 0.00152–0.00571 | |
| IIV CL (shrinkage 25%) | % | 22.4 | 18.1 | 18.2–25.7 |
| V1 = V1BW× V1RAC×Θ× (BW/70)1 | l | 4.42 | 2.35 | 4.21–4.64 |
| V1BW = 1 +Θ× (BW – 74.5) | −0.00354 | 23.1 | −0.00549 to −0.00178 | |
| Non-Asian: V1RAC = 1 | ||||
| Asian: V1RAC = 1 +Θ | −0.16 | 22.9 | −0.228 to −0.091 | |
| Q2 = Θ× (BW/70)0.75 | l min−1 | 0.206 | 4.69 | 0.188–0.225 |
| V2 = V2CR×Θ× (BW/70)1 | l | 6.35 | 2.82 | 6.00–6.71 |
| V2CR = Exp[Θ× (CR – 119)] | −0.00305 | 18.3 | −0.0041 to −0.0020 | |
| Residual error (shrinkage 3%) | % | 36.3 | 17.7 | 30.1–42.0 |
| Pharmacokinetic model complex | ||||
| kd | µm | 0.0559 | Fixed | |
| loge(k2) | min−1 | −3.38 | 16.5 | −4.20 to −1.43 |
| Pharmacodynamic model rocuronium | ||||
| keo = keoSEV×Θ× (BW/70)−0.25 | min−1 | 0.134 | 6.49 | 0.118–0.153 |
| No sevoflurane: keoSEV = 1 | ||||
| Sevoflurane: keoSEV = 1 +Θ | −0.567 | 8.94 | −0.668 to −0.467 | |
| IIV keo (shrinkage 0%) | % | 41.7 | 14.8 | 35.1–47.2 |
| EC50 = EC50SEV×Θ | µm | 1.62 | 3.68 | 1.50–1.75 |
| No sevoflurane: EC50SEV = 1 | ||||
| Sevoflurane: EC50SEV = 1 +Θ | −0.395 | 12.0 | −0.476 to −0.293 | |
| IIV EC50 (shrinkage 0%) | % | 24.9 | 16.9 | 19.9–28.4 |
| r (correlation keo and EC50) | 0.37 | 32.9 | 0.191–0.455 | |
| Hill = Θ | 7.52 | 5.84 | 6.99–8.15 | |
| IIV Hill (shrinkage 2%) | % | 41.1 | 22.0 | 32.7–48.5 |
| E0 = Θ× 100 | T4/T1 | 1.04 | 1.51 | 1.01–1.07 |
| IIV E0 (shrinkage 2%) | % | 11.1 | 21.5 | 8.5–13.3 |
| Residual error (shrinkage 5%) | T4/T1 | 2.70 | 7.03 | 2.50–2.87 |
| Pharmacodynamic model sugammadex | ||||
| ks = Exp(Θ) × (BW/70)−0.25 | min−1 µm−1 | −3.43 | 0.222 | −3.57 to −2.68 |
| IIV ks (shrinkage 3%) | % | 114% | 14.9 | 0.966–1.90 |
| Residual error (shrinkage 0%) | T4/T1 | 3.24 | 4.39 | 3.12–3.45 |
Pharmacokinetic and PK–PD parameters are as presented in Figure 1. RSE(%), relative standard error describing uncertainty in the parameter (100 × SE/population estimate). SE, asymptotic standard error estimate of the parameter (taken from $COV of NONMEM). IIV, interindividual variability. CI, confidence interval. Parameterization complex formation kd = k2/k1. SEV, sevoflurane. RAC, race. BW, bodyweight, CR, creatinine clearance. Emax for rocuronium NMB is set equal to E0.
Pharmacokinetic interaction model for rocuronium and sugammadex
Observed and population-predicted sugammadex plasma concentrations vs. time by dose are presented in Figure 2. A two-compartmental model was applied for both compounds and for the complex. The three-compartmental model was less stable and became very time consuming when covariates were included. The two-compartmental model adequately described the data within the clinically relevant concentration range.
Figure 2.
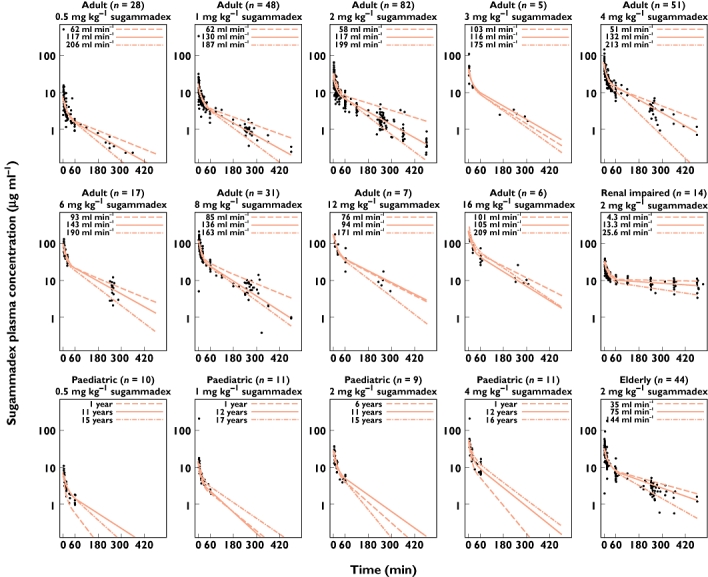
Observed and population-predicted sugammadex plasma concentrations for adult, paediatric, elderly and renally impaired populations conditioned on sugammadex dose (one subject treated with sugammadex 0.1 mg kg−1 is not shown). Red lines represent population predictions for three typical subjects reflecting variability in renal function for adults or age for paediatric subjects. Demographic data from patients with lowest, median and highest creatinine clearance, or age in case of the paediatric population, within the depicted populations were used to define typical subjects; all data are shown up to 10 h postdose
Population prediction concentration vs. time profiles were in good agreement with observed data. The covariate creatinine clearance as a predictor for renal function in adults and elderly, and age in the paediatric subset, explains a large part of the variability in the sugammadex concentrations. Goodness-of-fit plots for sugammadex conditioned for bodyweight of the final PK model are represented in Figure 3. Concentrations were predicted adequately in the Asian, paediatric, elderly and renally impaired populations (data not shown). Conditional weighted residual and individual weighted residuals were randomly distributed over time and over the predicted concentration range. Rocuronium plasma concentrations were equally well described by the model.
Figure 3.
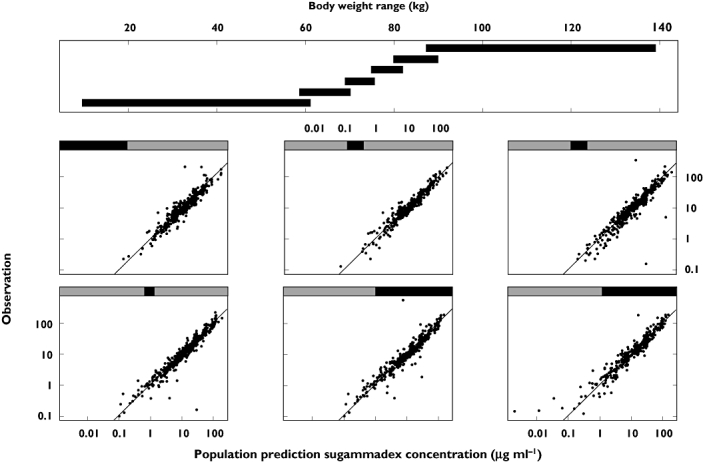
Observed sugammadex concentrations vs. population predictions split on bodyweight
Allometric scaling in general addressed size effects well. Only for sugammadex V1s, effects of bodyweight were less pronounced than expected from the allometric approach. For the average subject (non-Asian, aged 43 years, bodyweight 74.5 kg, creatinine clearance 119 ml min−1), covariate effects were quantified as follows: on top of the allometrical scaling, a 10 kg increase in bodyweight resulted in a 3.8% increase in sugammadex CLs and a 3.5% decrease in sugammadex V1s; a 10 year increase in age resulted in a rocuronium CLr decrease of 6.8% and a rocuronium V2r increase of 6.2%; and a 10 ml min−1 increase in creatinine clearance decreased rocuronium V1r by 1.4% and sugammadex V2s by 3.1%. Effects of age, taking into account renal function and bodyweight, on sugammadex clearance for typical subjects as defined in Table 4 are illustrated in Figure 4. Effects of race were limited to rocuronium Q2r, being 21% lower in Asians, and sugammadex V1s, being 16% lower in Asians. No effect of gender on any of the PK parameters was found. Shrinkage of PK parameters was moderate, ranging from 12 to 37%. Epsilon shrinkage was 14% for rocuronium and 3% for sugammadex.
Figure 4.
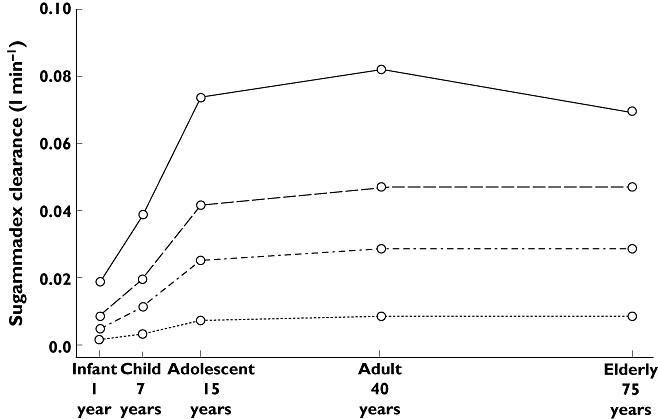
Sugammadex clearance in the paediatric, adult and elderly population for various stages of renal function (as defined in Table 4). Normal renal function ( ); Mild renal impairment (
); Mild renal impairment ( ); Moderate renal impairment (
); Moderate renal impairment ( ); Severe renal impairment (
); Severe renal impairment ( )
)
The dynamic interaction model performed better than the instantaneous model. Complexation was characterized by an estimated k2 of 0.034 min−1; as a result, k1 was 0.61 min−1 (kd = k2/k1, with kd fixed at 0.0559 µm). The association and dissociation half-lives were 1.1 and 20.4 min, respectively.
Bootstrap results confirmed the parameter estimates, except for k2, where a biphasic distribution with a small secondary minimum at 0.22 min−1 was found, indicative for a faster on- and off-rate for complexation.
Pharmacokinetic–pharmacodynamic model for rocuronium-induced NMB
Observed and population-predicted T4/T1 twitch ratio profiles after rocuronium 0.6 or 1.2 mg kg−1 in the absence of sevoflurane anaesthesia, or after rocuronium 0.9 mg kg−1 in the presence of sevoflurane anaesthesia, are given in Figure 5.
Figure 5.
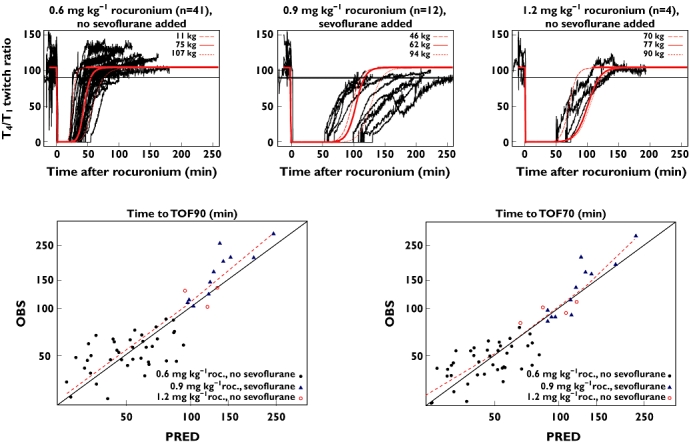
Upper graphs show the observed T4/T1 twitch ratio upon spontaneous neuromuscular blockade reversal following rocuronium administration, conditioned on administered dose and use of sevoflurane anaesthesia (n = 59). Lower graphs show the posterior predictive check of the pharmacokinetic–pharmacodynamic model for spontaneous reversal [time to train-of-four ratios of 0.7 and 0.9 (TOF70 and TOF90)] of rocuronium-induced neuromuscular blockade, observed (OBS) vs. predicted (PRED) reversal times. Red lines represent population predictions for three typical subjects reflecting variability in bodyweight. Demographic data from patients with lowest, median and highest bodyweight, within the depicted populations were used to define these typical subjects; two patients receiving maintenance rocuronium doses are not shown. Red dashed lines in lower graphs represent loess smooth curve. Prediction error (OBS–PRED) for population-predicted TOF90 was as follows: 1.5 min for 0.6 mg kg−1 rocuronium with no sevoflurane; 23.25 min for 0.9 mg kg−1 rocuronium with sevoflurane; and 1.25 min for 1.2 mg kg−1 rocuronium with no sevoflurane. Prediction error (OBS–PRED) for population-predicted TOF70 was as follows: 0.88 min for 0.6 mg kg−1 rocuronium with no sevoflurane; 6.75 min for 0.9 mg kg−1 rocuronium with sevoflurane; and1.63 min for 1.2 mg kg−1 rocuronium with no sevoflurane
Sevoflurane effects were included for keo and EC50. The estimated keo was 0.134 min−1, and if sevoflurane was used the keo decreased to 0.058 min−1; corresponding half-lives were 5.2 and 12 min, respectively. Likewise, the estimated EC50 decreased from 1.62 to 0.98 µm when sevoflurane anaesthesia was applied, demonstrating the 40% potentiation of rocuronium under sevoflurane anaesthesia. Allometric scaling of the rate constant keo resulted in a five-point drop in OFV and a 10% reduction in IIV on keo (data not shown). Residual bodyweight or age effects were not found for keo. Shrinkage in PD parameters and epsilon was low (≤5%).
Population-predicted T4/T1 twitch ratio profiles were in line with observed profiles. Although rocuronium administration was bodyweight adjusted, variability in recovery between patients who were not treated with sevoflurane can, in large part, be explained by bodyweight effects. In cases where sevoflurane was being used as the anaesthetic agent, variability in recovery between subjects increased. Posterior predictive checking for the PK–PD model for rocuronium is illustrated in Figure 5. In general, reversal times after rocuronium-induced NMB were well predicted when no sevoflurane was applied as anaesthetic agent. In cases where sevoflurane was applied, the model was less accurate in predicting the time to TOF90. The prediction error for the population-predicted reversal time was 23.25 min, with observed time to TOF90 ranging between 103 and 297 min after 0.9 mg kg−1 rocuronium. For the reversal time to TOF70, bias was less pronounced, the prediction error being 6.75 min.
Pharmacokinetic–pharmacodynamic model for sugammadex-mediated reversal of rocuronium-induced NMB
The estimated ks was 0.033 min−1 µm−1 free sugammadex in the central compartment for a patient with bodyweight of 70 kg. Immediately after administration of sugammadex 2 or 4 mg kg−1, the rocuronium elimination half-life from the effect compartment was calculated to be 1.43 and 0.71 min, respectively. For comparison, without sugammadex, the half-life of rocuronium from the effect compartment was 5.2 min (keo = 0.134 min−1). Effects of sevoflurane on sugammadex-mediated reversal of rocuronium-induced NMB were not found. Allometric scaling of the rate constant ks was included (ΔOFV = − 10), resulting in a slight reduction in between-subject variability. No residual age or bodyweight effects were found, confirming the validity of allometric scaling. The IIV was high (114%), but in line with variability in clinical observations. Shrinkage in PD parameters and epsilon was low (≤3%).
Posterior predictive checking for the PK–PD model for sugammadex is illustrated in Figure 6, showing observed and predicted reversal times to TOF90 and TOF70. Population-predicted reversal times are in line with observed reversal times. Prediction errors for time to TOF90 and time to TOF70 were 0.25 and 0 min, respectively. Individual predictions for time to TOF90 were 0.5 min faster than observed reversal times. In addition, individual predicted times to TOF90 were faster than observed times to TOF90 in 80% of the patients. The TOF90 times were more variable than TOF70 times.
Figure 6.
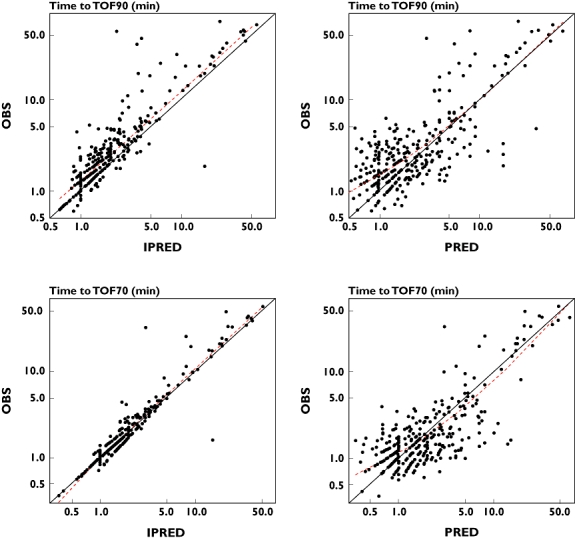
Posterior predictive check of the pharmacokinetic–pharmacodynamic model for sugammadex mediated reversal times (time to train-of-four ratio of 0.9 [TOF90] and 0.7 [TOF70]) of rocuronium-induced neuromuscular blockade, observed (OBS) and predicted (PRED and IPRED) reversal times. Red dashed lines represent loess smooth curve. Prediction error (OBS-PRED) for population-predicted TOF90 time: 0.25 min; Prediction error (OBS-IPRED) for individual predicted TOF90 time: 0.5 min; Prediction error (OBS-PRED) for population-predicted TOF70 time: 0 min; Prediction error (OBS-IPRED) for individual predicted TOF70 time: 0 min
Performance of the model in various scenarios, upon several sugammadex dosages and in various age classes is illustrated in Figure 7. In general, time to TOF90 is somewhat underpredicted, in line with the observed prediction error. When sugammadex was administered at fixed time points, after either 0.6 or 1.2 mg kg−1 rocuronium, observed and predicted TOF90 reversal times were in good agreement over the whole dose range except for the 8 mg kg−1 sugammadex dose given at 5 min after 1.2 mg kg−1 rocuronium. For most of the moderate block reversal scenarios, observed and predicted TOF90 times largely overlapped. Predictions for the therapeutic 2 mg kg−1 sugammadex dose for moderate blockade reversal were in line with observed data, both in the presence and the absence of sevoflurane anaesthesia and for renally impaired patients. In the presence of sevoflurane anaesthesia, TOF90 times were predicted well for all sugammadex dose levels. In the absence of sevoflurane anesthesia, slower TOF90 times were predicted upon 1 mg kg−1 sugammadex and faster reversal was predicted upon 4 mg kg−1 sugammadex. In general, upon higher sugammadex dose levels, a tendency for faster, more efficient, predicted reversal was observed compared with the observed reversal times. Performance of the model for various age classes indicated that predictions and observed TOF90 were in agreement for the paediatric age classes and adults, but the trend of slower recovery observed in the elderly (≥75 years) subset was not retained in the model.
Figure 7.
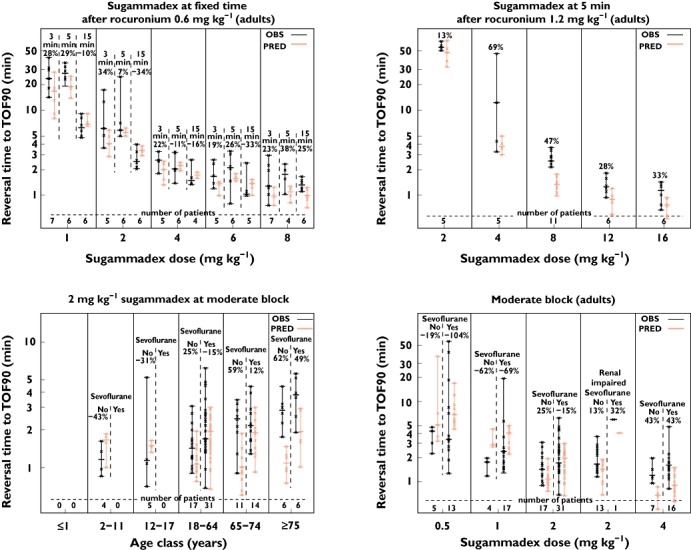
Posterior predictive check of the pharmacokinetic–pharmacodynamic model for sugammadex-mediated reversal times [time to train-of-four ratio of 0.9 (TOF90)] of rocuronium-induced neuromuscular block, observed (OBS) and predicted (PRED) reversal times. Crosses represent individual values, bars represent range and horizontal lines represent the median. Relative difference between reversal times [(OBS–PRED)/OBS × 100] is presented above the bars
External validation
Table 5 shows the predicted and observed reversal times for the various treatment groups of clinical trial 19.4.207 (P05930) [13] and clinical trial 19.4.210 (P05959) [14].
Table 5.
External validation with clinical trials 19.4.207 (P05930) [13] and 19.4.210 (P05959) [14], showing time to recovery of the train-of-four (TOF) ratio to 0.9 (TOF90) and 0.7 (TOF70), following sugammadex reversal*
| Clinical trial | 19.4.207 (P05930) | 19.4.210 (P05959) | ||||||
|---|---|---|---|---|---|---|---|---|
| Anaesthesia | Propofol | Propofol | Sevoflurane | |||||
| End-point | Dose statistics | 0.5 mg kg−1sugammadex (n = 8) | 1.0 mg kg−1sugammadex (n = 7) | 2.0 mg kg−1sugammadex (n = 8) | 3.0 mg kg−1sugammadex (n = 3) | 4.0 mg kg−1sugammadex (n = 8) | 2.0 mg kg−1sugammadex (n = 21) | 2.0 mg kg−1sugammadex (n = 20) |
| Time to TOF90 (min) | Simulated median | 4.6 | 2.5 | 1.5 | 1.0 | 0.8 | 1.5 | 1.5 |
| Simulated 90% CI | 1.5–15 | 0.8–7.6 | 0.5–5.5 | 0.3–4.3 | 0.3–3.5 | 0.5–5.5 | 0.5–8.0 | |
| Observed median | 3.7 | 2.2 | 1.7 | 1.5 | 1.1 | 1.7 | 1.7 | |
| Observed range | 2.1–4.9 | 1.5–3.4 | 0.9–2.8 | 1.0–3.2 | 0.7–1.6 | 0.9–3.4 | 1.1–4.5 | |
| Time to TOF70 (min) | Simulated median | 3.5 | 2.0 | 1.3 | 0.8 | 0.8 | 1.1 | 1.3 |
| Simulated 90% CI | 1.2–11 | 0.7 −6.0 | 0.5–4.3 | 0.3–3.5 | 0.3–2.8 | 0.5–4.3 | 0.5–6.0 | |
| Observed median | 2.4 | 1.4 | 1.3 | 1.0 | 1.0 | 1.2 | 1.3 | |
| Observed range | 1.3–2.9 | 1.1–2.2 | 0.9–1.9 | 0.7–2.4 | 0.7–1.3 | 0.8–2.4 | 0.7–1.9 | |
Sugammadex was administered at reappearance of T2 (moderate blockade reversal).
For all scenarios, median reversal times to TOF90 were well predicted, being within the range of the observed reversal times. Median predicted reversal time to TOF90 for the 2 mg kg−1 sugammadex dose, which is the therapeutic dose for reversal of moderate blockade, was 1.5 min, independent of whether propofol or sevoflurane anaesthesia was applied. Predicted TOF90 time was 0.2 min faster than the observed reversal time, but predicted time to TOF70 was equivalent to the observed median reversal time, which is in line with the observed prediction error in the posterior predictive checking plots. The trend of decreasing reversal times with increasing sugammadex doses was also preserved in the simulated reversal times. In comparison with the observed dose–response curve, the model-predicted dose–response curve showed a slight tendency of slower simulated reversal times at lower dose levels and faster reversal times at higher dose levels. Simulated TOF70 reversal times showed a similar pattern; in general, predicted TOF70 reversal times were closer to the observed reversal time.
Simulated 90% confidence intervals in all scenarios were wider than expected from the clinical data. This was most probably caused by the sequential approach of model development, using post hoc estimates for subsequent modelling steps, thereby excluding the possibility of retaining information on correlation between PK parameters, rocuronium NMB and sugammadex NMB parameters.
Simulations
The model-predicted effects of age and sevoflurane use on reversal times upon reversal 3 min after rocuronium or during deep or moderate blockade reversal are illustrated in Table 6. Simulated reversal times in adults were 0.8, 1.5 and 1.4 min upon reversal 3 min after rocuronium with sugammadex 16 mg kg−1, deep blockade reversal with sugammadex 4 mg kg−1 and moderate block reversal with 2 mg kg−1, respectively. Upon reversal 3 min after rocuronium, predicted reversal times were within 1 min in all cases; this was also observed when sevoflurane anaesthesia was applied. Age effects were hardly visible. Upon reversal of deep blockade, age effects were more apparent. In the paediatric age range, reversal times were up to almost twofold faster compared with adults, while in the elderly population, slightly slower (≤0.3 min) reversal times were found compared with adults. Sevoflurane anaesthesia increased the reversal time by 0.3 min in the adult population. Upon moderate blockade reversal, similar, but less pronounced, effects of age and sevoflurane anaesthesia were found. Renal function did not affect simulated reversal time in any of the simulated scenarios (data not shown). Uncertainty was low; for the deep block reversal in adults, the coefficient of variation was 3.2%. Despite the wide range of covariates, and also taking the observed bias into account, the predicted median reversal time for each simulated scenario stayed within 2 min.
Table 6.
Simulated reversal times [time to train-of-four ratio to 0.9 (TOF90), median and 90% confidence interval] for typical non-Asian subjects with normal renal function
| Reversal time to TOF90 (min) | ||||||
|---|---|---|---|---|---|---|
| Anaesthesia | No sevoflurane | Sevoflurane | ||||
| Scenario | Infant 1 year | Child 7 years | Adolescent 15 years | Adult 40 years | Elderly 75 years | Adult 40 years |
| Reversal 3 min after rocuronium | 0.5 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| 16 mg kg−1 sugammadex at 3 min after 1.2 mg kg−1 rocuronium | 0.3–2.5 | 0.3–2.8 | 0.3–3.3 | 0.3–3.3 | 0.3–3.5 | 0.3–3.5 |
| Deep blockade reversal | 0.8 | 1.0 | 1.3 | 1.5 | 1.8 | 1.8 |
| 4 mg kg−1 sugammadex at 15 min after 0.6 mg kg−1 rocuronium | 0.3–3.3 | 0.3–4.3 | 0.3–5.5 | 0.5–6.0 | 0.5–6.5 | 0.5–11 |
| Moderate blockade reversal | 1.0 | 1.3 | 1.3 | 1.4 | 1.5 | 1.6 |
| 2 mg kg−1 sugammadex at reappearance of T2 | 0.3–3.8 | 0.5–4.8 | 0.5–5.5 | 0.5–5.5 | 0.5–6.0 | 0.5–8.5 |
Discussion
In summary, the newly developed PK and PK–PD model further extended the previous modelling approaches. Rocuronium and sugammadex PK and their mutual interaction were modelled simultaneously. The full PK interaction model and subsequent rocuronium and sugammadex PK–PD models were updated and established on a broader data set including paediatric and elderly populations, non-Asian and Asian populations, as well as patients with varying degrees of renal impairment, allowing for an appropriate covariate evaluation.
To account for the large range in body size of the population (aged 1–91 years), PK parameters and rate constants in PD models were allometrically scaled, except for the size effects on sugammadex clearance, which were for the larger part addressed by renal function. On top of these structural effects, only limited linear bodyweight effects were found for clearance and central volume of distribution of sugammadex, confirming the validity of the structural model. The rocuronium PK model has an age-dependent linear decrease in clearance in elderly, but not in younger patients. The clearance is dependent on age (linear) and bodyweight (power/allometry). Bodyweight is the driving component in paediatrics. Age has only a limited impact, potentially due to standard allometric scaling factors. However, when testing model-estimated factors, overparameterization was observed, probably due to the high correlation between age and bodyweight. Thus, a size-corrected elimination of rocuronium in paediatrics best described the available data.
Two-compartmental models, instead of the previously applied three-compartmental models, for rocuronium, sugammadex and the complex were sufficient to describe the concentrations in the therapeutic concentration range. A two-compartmental model for rocuronium PK has been applied previously [23, 24, 40]. The bias observed at the extremes of the rocuronium concentration range, at a large distance from the EC50, does not translate to effects in NMB and was therefore judged clinically irrelevant and acceptable.
Sugammadex plasma concentration-mediated modulation of the rocuronium elimination from the effect compartment provides an adequate framework to describe the fast sugammadex effects on NMB and is, on a physiological level, well in line with the underlying processes [3]. On a structurally mechanistic level, allometrical scaling of the rate constant for sugammadex-mediated elimination of rocuronium from the effect compartment managed the faster reversal as observed in the paediatric population. However, the model was less well able to address the somewhat slower reversal in the geriatric population. Variability in reversal time could, to some extent, be addressed with allometric scaling, but a substantial part of variability remained unidentified. A measure for cardiac output, reflecting the efficiency of the body in transferring sugammadex to the site of action, and/or the use of a recirculatory PK model (involving a nondistributive pathway) could contribute to explaining unidentified variability [41] and the somewhat slower reversal in elderly patients. Unfortunately, a measure for cardiac output was not available in this analysis. In addition, application of a recirculatory PK model requires technically demanding experiments, making it unsuitable for routine measurement.
In scenarios where relatively high rocuronium exposure exists (sugammadex administration very soon after rocuronium), the model tends to predict a more efficient reversal than actually observed (0.8 vs. 1.3 min in clinical trial 19.4.205 (P05942) [7]). The discrepancy between observed and predicted reversal to full recovery could point to a second deeper, less perfused NMB compartment which is not incorporated in the current model.
Individual predicted reversal times to TOF90 were on average 0.5 min faster than observed reversal time (Figures 6 and 7). The observed bias could be linked to disturbances in the baseline T4/T1 twitch ratio, e.g. movement of the patient, resulting in a less optimal configuration of the assessment of NMB. As a consequence, the T4/T1 twitch ratio at full recovery (baseline) may become <1, and attainment of the reversal is delayed, as illustrated in Figure 8, or is not attained at all. The impact on time to TOF90 is more pronounced than the effect on time to TOF70.
Figure 8.
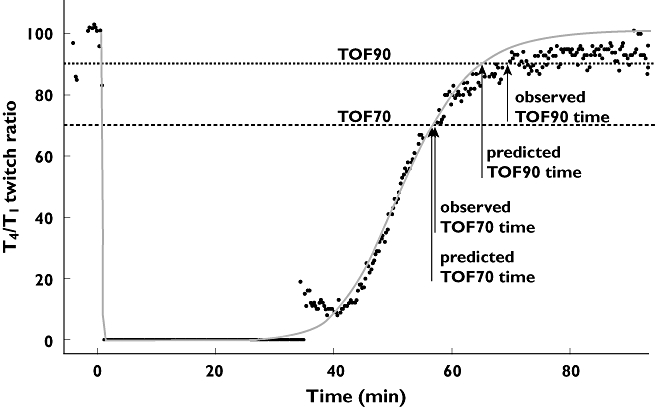
Bias in predicted time to a train-of-four ratio of 0.9 (TOF90) reversal due to disturbances in the baseline T4/T1 twitch ratio. Observed and individual fit of T4/T1 twitch profile after 0.6 mg kg−1 rocuronium, including assessment of TOF70 and TOF90 reversal times
Results from the external validation showed that the action of sugammadex on reversing rocuronium-induced NMB was predicted well. Predicted variability in reversal times was, however, higher than observed.
Clinical data [12] in paediatric patients demonstrated that age effects on sugammadex-mediated moderate blockade reversal were limited. After 2.0 mg kg−1 sugammadex, TOF90 was attained in 0.6, 1.2, 1.1 and 1.2 min in infants, children, adolescents and adults, respectively. Model-based predictions confirm these observations and also predict that age effects were more pronounced after sugammadex-mediated deep blockade reversal. Mechanistically, these observations are plausible, taking into account that distribution processes occur faster in subjects with lower distribution volumes. Rocuronium concentrations are higher at initiation of reversal at deep blockade than at moderate blockade (reappearance of T2). Hence, more sugammadex is needed, leading to a more pronounced age-related differentiation in reversal time. In cases of reversal with 16 mg kg−1 sugammadex given 3 min after rocuronium, the excess of sugammadex renders the limited age effects to nil.
Simulations indicate that renal function, in line with clinical observations [10], does not affect reversal time. The sugammadex elimination half-life in plasma, increasing from 2 h in healthy subjects to 20 h in severe renally impaired subjects, is too slow to have any impact on reversal time; in contrast, the half-life for sugammadex-mediated elimination of rocuronium from the effect compartment is <2 min. Experiments performed in anaesthetized cats, in which both renal arteries were completely occluded, support these findings [42].
In general, the model-predicted covariate effects are limited and not clinically relevant. In simulations applying the therapeutic dosing regimens, the reversal time for typical subjects remained below 2 min (also when taking into account the observed bias of 0.25 min), thereby confirming the clinically recommended dose regimens [43].
It is concluded that the final PK–PD model provides a solid framework, based on physiological principles and on data covering a wide range for age (1–91 years) and renal functioning (creatinine clearance range 4.3–221 ml min−1), from which simulations can be made to predict effects of sugammadex in unexplored regimens or populations that were not investigated.
Acknowledgments
Editorial support was provided by Valerie Moss PhD (Prime Medica Ltd, Knutsford, UK) during later stages of manuscript development. This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, USA. The design and conduct of the study, as well as analysis of the study data and opinions, conclusions and interpretation of the data, were the responsibility of the authors.
Competing Interests
All authors are employees of Merck Sharp & Dohme Corp., Merck Research Laboratories, MSD, Oss, The Netherlands. TK owns shares/options in Merck Inc.
REFERENCES
- 1.Bevan DR. Monitoring and reversal of neuromuscular block. Am J Health Syst Pharm. 1999;56(Suppl. 1):S10–13. doi: 10.1093/ajhp/56.S10. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava A, Hunter JM. Reversal of neuromuscular block. Br J Anaesth. 2009;103:115–29. doi: 10.1093/bja/aep093. [DOI] [PubMed] [Google Scholar]
- 3.Bom A, Hope F, Rutherford S, Thomson K. Preclinical pharmacology of sugammadex. J Crit Care. 2009;24:29–35. doi: 10.1016/j.jcrc.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Gijsenbergh F, Ramael S, Houwing N, van Iersel T. First human exposure of Org 25969, a novel agent to reverse the action of rocuronium bromide. Anesthesiology. 2005;103:695–703. doi: 10.1097/00000542-200510000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Sorgenfrei IF, Norrild K, Larsen PB, Stensballe J, Ostergaard D, Prins ME, Viby-Mogensen J. Reversal of rocuronium-induced neuromuscular block by the selective relaxant binding agent sugammadex: a dose-finding and safety study. Anesthesiology. 2006;104:667–74. doi: 10.1097/00000542-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Sparr HJ, Vermeyen KM, Beaufort AM, Rietbergen H, Proost JH, Saldien V, Velik-Salchner C, Wierda JM. Early reversal of profound rocuronium-induced neuromuscular blockade by sugammadex in a randomized multicenter study: efficacy, safety, and pharmacokinetics. Anesthesiology. 2007;106:935–43. doi: 10.1097/01.anes.0000265152.78943.74. [DOI] [PubMed] [Google Scholar]
- 7.de Boer HD, Driessen JJ, Marcus MA, Kerkkamp H, Heeringa M, Klimek M. Reversal of rocuronium-induced (1.2 mg/kg) profound neuromuscular block by sugammadex: a multicenter, dose-finding and safety study. Anesthesiology. 2007;107:239–44. doi: 10.1097/01.anes.0000270722.95764.37. [DOI] [PubMed] [Google Scholar]
- 8.Schering-Plough. A Bridging Trial Comparing Org 25969 at Reappearance of T2 in Japanese and Caucasian Subjects. Part A: Japanese Subjects (19.4.208A) (P05956)(COMPLETED). Date last updated: October 2, 2009. Available at http://www.clinicaltrials.gov/ct2/show/NCT00591409 (last accessed 20 July 2010)
- 9.Pühringer FK, Gordon M, Demeyer I, Sparr HJ, Ingimarsson J, Klarin B, van Duijnhoven W, Heeringa M. Sugammadex rapidly reverses moderate rocuronium- or vecuronium-induced neuromuscular block during sevoflurane anaesthesia: a dose-response relationship. Br J Anaesth. 2010;105:610–9. doi: 10.1093/bja/aeq226. [DOI] [PubMed] [Google Scholar]
- 10.Staals LM, Snoeck MM, Driessen JJ, van Hamersvelt HW, Flockton EA, van den Heuvel MW, Hunter JM. Reduced clearance of rocuronium and sugammadex in patients with severe to end-stage renal failure: a pharmacokinetic study. Br J Anaesth. 2010;104:31–9. doi: 10.1093/bja/aep340. [DOI] [PubMed] [Google Scholar]
- 11.McDonagh DL, Benedict PE, Kovac AL, Drover DR, Brister NW, Morte JB, Monk TG. Efficacy, safety, and pharmacokinetics of sugammadex for the reversal of rocuronium-induced neuromuscular blockade in elderly patients. Anesthesiology. 2011;114:318–29. doi: 10.1097/ALN.0b013e3182065c36. [DOI] [PubMed] [Google Scholar]
- 12.Plaud B, Meretoja O, Hofmockel R, Raft J, Stoddart PA, van Kuijk JH, Hermens Y, Mirakhur RK. Reversal of rocuronium-induced neuromuscular blockade with sugammadex in pediatric and adult surgical patients. Anesthesiology. 2009;110:284–94. doi: 10.1097/ALN.0b013e318194caaa. [DOI] [PubMed] [Google Scholar]
- 13.Suy K, Morias K, Cammu G, Hans P, van Duijnhoven WG, Heeringa M, Demeyer I. Effective reversal of moderate rocuronium- or vecuronium-induced neuromuscular block with sugammadex, a selective relaxant binding agent. Anesthesiology. 2007;106:283–8. doi: 10.1097/00000542-200702000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Vanacker BF, Vermeyen KM, Struys MM, Rietbergen H, Vandermeersch E, Saldien V, Kalmar AF, Prins ME. Reversal of rocuronium-induced neuromuscular block with the novel drug sugammadex is equally effective under maintenance anesthesia with propofol or sevoflurane. Anesth Analg. 2007;104:563–8. doi: 10.1213/01.ane.0000231829.29177.8e. [DOI] [PubMed] [Google Scholar]
- 15.Blobner M, Eriksson LI, Scholz J, Motsch J, Della Rocca G, Prins ME. Reversal of rocuronium-induced neuromuscular blockade with sugammadex compared with neostigmine during sevoflurane anaesthesia: results of a randomised, controlled trial. Eur J Anaesthesiol. 2010;27:874–81. doi: 10.1097/EJA.0b013e32833d56b7. [DOI] [PubMed] [Google Scholar]
- 16.Jones RK, Caldwell JE, Brull SJ, Soto RG. Reversal of profound rocuronium-induced blockade with sugammadex: a randomized comparison with neostigmine. Anesthesiology. 2008;109:816–24. doi: 10.1097/ALN.0b013e31818a3fee. [DOI] [PubMed] [Google Scholar]
- 17.Lee C, Jahr JS, Candiotti KA, Warriner B, Zornow MH, Naguib M. Reversal of profound neuromuscular block by sugammadex administered three minutes after rocuronium: a comparison with spontaneous recovery from succinylcholine. Anesthesiology. 2009;110:1020–5. doi: 10.1097/ALN.0b013e31819dabb0. [DOI] [PubMed] [Google Scholar]
- 18.Peeters P, Passier P, Smeets J, Zwiers A, de Zwart M, van Marle S. Sugammadex is cleared rapidly and primarily in an unchanged form via renal excretion. Clinical. Pharmacol Ther. 2009;85(Suppl. 1):S83. doi: 10.1002/bdd.747. Abstract PIII-50. [DOI] [PubMed] [Google Scholar]
- 19.Peeters PA, van den Heuvel MW, van Heumen E, Passier PC, Smeets JM, van Iersel T, Zwiers A. Safety, tolerability and pharmacokinetics of sugammadex using single high doses (up to 96 mg/kg) in healthy adult subjects: a randomized, double-blind, crossover, placebo-controlled, single-centre study. Clin Drug Investig. 2010;30:867–74. doi: 10.1007/BF03256915. [DOI] [PubMed] [Google Scholar]
- 20.Ploeger BA, Smeets J, Strougo A, Drenth HJ, Ruigt G, Houwing N, Danhof M. Pharmacokinetic-pharmacodynamic model for the reversal of neuromuscular blockade by sugammadex. Anesthesiology. 2009;110:95–105. doi: 10.1097/ALN.0b013e318190bc32. [DOI] [PubMed] [Google Scholar]
- 21.Bock M, Klippel K, Nitsche B, Bach A, Martin E, Motsch J. Rocuronium potency and recovery characteristics during steady-state desflurane, sevoflurane, isoflurane or propofol anaesthesia. Br J Anaesth. 2000;84:43–7. doi: 10.1093/oxfordjournals.bja.a013380. [DOI] [PubMed] [Google Scholar]
- 22.Lowry DW, Mirakhur RK, McCarthy GJ, Carroll MT, McCourt KC. Neuromuscular effects of rocuronium during sevoflurane, isoflurane, and intravenous anesthesia. Anesth Analg. 1998;87:936–40. doi: 10.1097/00000539-199810000-00036. [DOI] [PubMed] [Google Scholar]
- 23.Woloszczuk-Gebicka B, Wyska E, Grabowski T, Swierczewska A, Sawicka R. Pharmacokinetic-pharmacodynamic relationship of rocuronium under stable nitrous oxide-fentanyl or nitrous oxide-sevoflurane anesthesia in children. Paediatr Anaesth. 2006;16:761–8. doi: 10.1111/j.1460-9592.2005.01840.x. [DOI] [PubMed] [Google Scholar]
- 24.Woloszczuk-Gebicka B, Wyska E, Grabowski T. Sevoflurane increases fade of neuromuscular response to TOF stimulation following rocuronium administration in children. A PK/PD analysis. Paediatr Anaesth. 2007;17:637–46. doi: 10.1111/j.1460-9592.2006.02181.x. [DOI] [PubMed] [Google Scholar]
- 25.de Zwart MAH, ten Bruggencate-Broeders J, van Hal HJM, Megens HJJJ, Frasa WLH. Determination of sugammadex in human plasma, urine, and dialysate using a high-performance liquid chromatography/tandem mass spectrometry assay. J Chromatogr B. 2011;879:1573–86. doi: 10.1016/j.jchromb.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs-Buder T, Claudius C, Skovgaard LT, Eriksson LI, Mirakhur RK, Viby-Mogensen J. 8th International Neuromuscular Meeting. Good clinical research practice in pharmacodynamic studies of neuromuscular blocking agents II: the Stockholm revision. Acta Anaesthesiol Scand. 2007;51:789–808. doi: 10.1111/j.1399-6576.2007.01352.x. [DOI] [PubMed] [Google Scholar]
- 27.Claudius C, Skovgaard LT, Viby-Mogensen J. Acceleromyography and mechanomyography for establishing potency of neuromuscular blocking agents: a randomized-controlled trial. Acta Anaesthesiol Scand. 2009;53:449–54. doi: 10.1111/j.1399-6576.2008.01866.x. [DOI] [PubMed] [Google Scholar]
- 28.Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit–A collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79:241–57. doi: 10.1016/j.cmpb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Wählby U, Jonsson EN, Karlsson MO. Assessment of actual significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn. 2001;28:231–52. doi: 10.1023/a:1011527125570. [DOI] [PubMed] [Google Scholar]
- 30.Wählby U, Bouw MR, Jonsson EN, Karlsson MO. Assessment of type I error rates for the statistical sub-model in NONMEM. J Pharmacokinet Pharmacodyn. 2002;29:251–69. doi: 10.1023/a:1020254823597. [DOI] [PubMed] [Google Scholar]
- 31.Hull CJ, Van Beem HBH, McLeod K, Sibbald A, Watson MJ. A pharmacodynamic model for pancuronium. Br J Anaesth. 1978;50:1113–23. doi: 10.1093/bja/50.11.1113. [DOI] [PubMed] [Google Scholar]
- 32.Sheiner LB, Stanski DR, Vozeh S, Miller RD, Ham J. Simultaneous modeling of pharmacokinetics and pharmacodynamics: application to d-tubocurarine. Clin Pharmacol Ther. 1979;25:358–71. doi: 10.1002/cpt1979253358. [DOI] [PubMed] [Google Scholar]
- 33.Rajagopalan P, Gastonguay MR. Population pharmacokinetics of ciprofloxacin in pediatric patients. J Clin Pharmacol. 2003;43:698–710. [PubMed] [Google Scholar]
- 34.Anderson BJ, Woollard GA, Holford NH. A model for size and age changes in the pharmacokinetics of paracetamol in neonates, infants and children. Br J Clin Pharmacol. 2000;50:125–34. doi: 10.1046/j.1365-2125.2000.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–32. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- 36.Meibohm B, Läer S, Panetta JC, Barrett JS. Population pharmacokinetic studies in pediatrics: issues in design and analysis. AAPS J. 2005;7:E475–87. doi: 10.1208/aapsj070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–90. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 39.DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–71. [Google Scholar]
- 40.McCoy EP, Mirakhur RK, Maddineni VR, Wierda JM, Proost JH. Pharmacokinetics of rocuronium after bolus and continuous infusion during halothane anaesthesia. Br J Anaesth. 1996;76:29–33. doi: 10.1093/bja/76.1.29. [DOI] [PubMed] [Google Scholar]
- 41.Henthorn TK, Krejcie TC, Avram MJ. Early drug distribution: a generally neglected aspect of pharmacokinetics of particular relevance to intravenously administered anesthetic agents. Clin Pharmacol Ther. 2008;84:18–22. doi: 10.1038/clpt.2008.107. [DOI] [PubMed] [Google Scholar]
- 42.Staals LM, de Boer HD, van Egmond J, Hope F, van de Pol F, Bom AH, Driessen JJ, Booij LH. Reversal of rocuronium-induced neuromuscular block by sugammadex is independent of renal perfusion in anesthetized cats. J Anesth. 2011;25:241–6. doi: 10.1007/s00540-010-1090-3. [DOI] [PubMed] [Google Scholar]
- 43.Abrishami A, Ho J, Wong J, Yin L, Chung F. Sugammadex, a selective reversal medication for preventing postoperative residual neuromuscular blockade. Cochrane Database Syst Rev. 2009;(4):CD007362. doi: 10.1002/14651858.CD007362.pub2. [DOI] [PubMed] [Google Scholar]
- 44.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC growth charts for the United States: methods and development. National Center for Health Statistics. Vital Health Stat 11. 2002;246:1–190. [PubMed] [Google Scholar]


