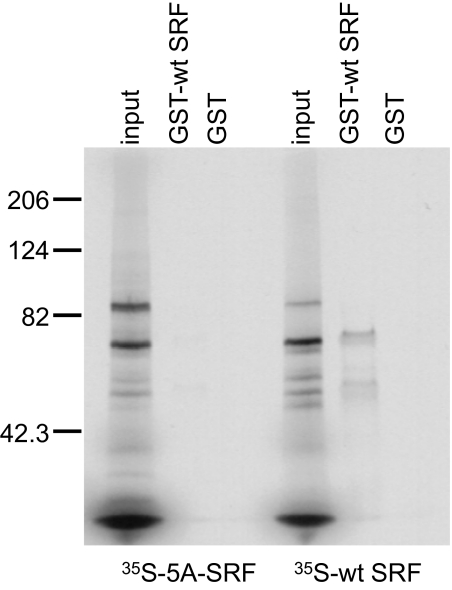
Figure 1.
Binding of the dimerization mutant 35S-5A serum response factor (SRF) to wild-type (wt)-SRF in solution is dramatically reduced but not eliminated. In vitro pull-down assay reveals that glutathione-S-transferase (GST)-wt SRF binds 35S-wt SRF much more avidly than it binds 35S-5A-SRF. Images are representative of at least two separate experiments and display the 35S-SRF species pulled down with GST-wt SRF. The smaller band in the GST-wt SRF lane represent nonspecific binding of 35S-wt SRF to the GST-wt SRF beads or breakdown products of 35S-wt SRF. GST-containing beads were used as the negative control for each pull-down assay. Five microliters of TNT transcription/translation solution, containing 35S-wt-SRF or 35S-5A-SRF proteins were used for each binding assay. Equal volumes (3 μl) of input proteins are shown for each binding assay.