Abstract
Inflammation plays a key role in the pathogenesis of bronchopulmonary dysplasia (BPD). Fatty acid–binding proteins (FABPs) 4 and 5 regulate the inflammatory activity of macrophages. Whether FABPs 4 and 5 could play a role in the pathogenesis of BPD via the promotion of macrophage inflammatory activity is unknown. This study sought to examine whether the expression levels of FABP4 and FABP5 were altered in bronchoalveolar lavage fluid and lung tissue in a baboon model of BPD. This study also sought to characterize the cell types that express these proteins. Real-time PCR, immunoblotting, immunohistochemistry, and double immunofluorescence were used to examine the expression of FABPs in samples of BPD. Morphometric analysis was used to quantify FABP4-positive peribronchial blood vessels in lung sections. FABP4 was primarily expressed in macrophages in samples of BPD. In addition, FABP4 was expressed in the endothelial cells of blood vessels in peribronchial areas and the vasa vasorum, but not in the alveolar vasculature in samples of BPD. FABP4 concentrations were significantly increased in lungs and bronchoalveolar lavage fluid samples with BPD. An increased density of FABP4-positive peribronchial blood vessels was evident in both baboon and human BPD sections. FABP5 was expressed in several cell types, including alveolar epithelial cells and macrophages. FABP5 concentrations did not show any significant alterations in BPD. In conclusion, FABP4 but not FABP5 levels are increased in BPD. FABP4 is differentially expressed in endothelial cells of the bronchial microvasculature, which demonstrates a previously unrecognized expansion in BPD.
Keywords: endothelium, macrophage, alveolarization, newborn, hyperoxia
Clinical Relevance
Inflammation and impaired angiogenesis play important roles in the pathogenesis of bronchopulmonary dysplasia (BPD). Fatty acid–binding proteins (FABPs) 4 and 5 regulate the inflammatory activity of macrophages. FABP4 is also expressed in a subset of endothelial cells, and is a target of the vascular endothelial growth factor (VEGF)/VEGF receptor 2 pathway. Whether FABPs are involved in the pathogenesis of BPD remains unknown. We found that FABP4 concentrations had significantly increased, whereas FABP5 concentrations had not altered, in bronchoalveolar lavage fluid and lung-tissue samples in a baboon model of BPD. FABP4 was detected in a subset of macrophages in BPD tissues. In addition, FABP4 was uniquely expressed in endothelial cells of the bronchial but not pulmonary microvasculature. A previously unrecognized expansion of FABP4-positive peribronchial blood vessels is evident in both baboon and human samples of BPD.
Bronchopulmonary dysplasia (BPD), a common complication of prematurity, develops in 30–40% of preterm infants with birth weights of less than 1,000 g (1). The etiology of BPD is multifactorial, with oxygen toxicity, ventilator-related trauma, and inflammation playing key roles (2–5). Infants with BPD are at higher risk for developing long-term pulmonary sequelae and other systemic problems, such as growth retardation and impaired neurodevelopment (6–9). Knowledge regarding the pathogenesis of BPD at the molecular level is limited, and effective therapeutic strategies for BPD are still lacking.
Fatty acid–binding proteins (FABPs) 4 and 5 belong to the family of highly homologous, low molecular weight, intracellular lipid-binding proteins (10–13). FABPs bind various hydrophobic ligands, such as long-chain fatty acids and retinoic acid, and are involved in the regulation of important biologic processes, including inflammation, lipid and glucose homeostasis, and cell proliferation and differentiation. The FABP family consists of nine proteins expressed with distinct tissue specificities, but with some overlap. FABP4, also known as adipocyte–FABP, was originally isolated from adipocytes, but was subsequently also detected in macrophages. We recently reported on the expression of FABP4 in a subset of endothelial cells (14). FABP5, also known as epidermal FABP, is the most widely expressed FABP, and is detected in a variety of cell types, including macrophages, epithelial cells, and endothelial cells.
Recent studies implicated FABP4 in the regulation of macrophage inflammatory activity. FABP4-deficient macrophages exhibit an impaired production of inflammatory cytokines and chemokines, such as IL-1β, TNFα, and monocyte chemotactic protein-1, in part because of enhanced peroxisome proliferator-activated receptor gamma (PPARγ) and reduced inhibitory kappa B (IκB) kinase activity (15). Consistent with these findings, Reynolds and colleagues reported lower levels of proinflammatory cytokine expression in the brain tissue of FABP4/FABP5 double knockout (FABP4/5−/−) mice in an experimental model of autoimmune encephalomyelitis (16). Inflammation is a major contributor to the pathogenesis of BPD, and FABP4-regulated proinflammatory cytokines are important biomarkers in the prediction of adverse pulmonary outcomes in preterm infants (17, 18). Furthermore, transcriptional pathways that are regulated by FABP4 (i.e., PPARγ and NF-κB) have been implicated in the pathogenesis of BPD (19–21). In rat and murine lungs, FABP5 was detected in alveolar epithelial Type 2 cells, fibroblasts, and macrophages (22, 23). FABP3/FABP5−/− mice demonstrate defective surfactant synthesis because of a decrease in uptake and incorporation of palmitic acid into glycerolipids, which can be ameliorated by a selective agonist of PPARγ (24).
The involvement of FABP4 and FABP5 in the regulation of inflammation suggests that they could play a role in the pathogenesis of BPD. Here, we investigated whether altered expression of FABP4 and FABP5 was associated with BPD, and examined the cell types that expressed these proteins in a well-characterized baboon model of BPD. We also used samples of human BPD to corroborate our findings, which demonstrate for the first time that the expression of FABP4 is uniquely confined to the bronchial circulation–derived vascular endothelial cells (ECs), and that this vascular bed undergoes an expansion during BPD.
Methods
Animal Models and Human Specimens
Baboon lung tissue samples were provided by the Southwest Foundation for Biomedical Research (SFBR) Primate Center (San Antonio, TX). Details of the baboon BPD model were described previously (25). All baboon procedures were approved by the Institutional Animal Care and Use Committee at the SFBR. Briefly, baboons that were delivered by hysterotomy at 125 days of gestation developed the characteristic features of the “new BPD” after surfactant replacement, mechanical ventilation, and pro re nata (PRN) oxygen treatment for 14 days (BPD group). Another group of baboons that were delivered at 140 days of gestation and killed immediately served as the gestational control group (140-day GC). Other control groups included baboons killed at 125 days of gestation or at full term (180 days). Discarded human autopsy specimens were obtained from the Department of Pathology at Boston Children's Hospital, with the approval of its Institutional Review Board.
RNA Isolation and Real-Time PCR
Total RNA was isolated from frozen proximal and distal baboon lung samples. Real-time PCR analysis was performed using the 2−ΔΔCT method, as previously described (14). Cyclophilin A was used as an internal reference to normalize the target transcripts. Real-time PCR primers are listed in Table E1 in the online supplement.
Immunoblot Analysis
Frozen lung tissue samples were homogenized in phosphate buffer, and immunoblotting was performed under reducing conditions, as previously described (26, 27).
Immunohistochemistry
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissue sections, as previously described (26). All primary antibody incubations were performed overnight at 4°C. The primary antibodies included rabbit polyclonal anti-FABP4 (Abcam, Cambridge, MA), diluted 1:200; murine monoclonal anti-FABP5 (R&D, Minneapolis, MN), diluted 1:50; monoclonal anti-CD31 (DAKO, Carpenteria, CA), diluted 1:50; monoclonal anti–α-smooth muscle actin (αSMA; Sigma, St. Louis, MO), diluted 1: 800; monoclonal anti-CD68, diluted 1:200 (DAKO); and monoclonal anti-CD163, diluted 1:100 (AbDSeroTec, Raleigh, NC). Antigen retrieval was performed for CD31 with Tris-EDTA buffer, pH 9, at 95°C for 15 minutes, and for CD68 and CD163 with citrate buffer, pH 6, at 95°C for 10 minutes. For double immunofluorescence, secondary antibodies included Alexa Fluor 594 goat anti-mouse IgG and Alexa Fluor 488 goat anti-rabbit IgG (Molecular Probes, Inc., Eugene, OR).
Morphometric Analysis
Peribronchial vessel density was analyzed in paraffin-embedded baboon and human lung samples immunostained for FABP4. Airways surrounded by hyaline cartilage that comprised 30–80% of their perimeters were included in the analysis. Images of airways and the surrounding area with FABP4-positive blood vessels were captured at ×200 magnification. Total numbers of vessels located between the inner surface of the cartilage and the airway lumen were quantified and then normalized to the perimeter of each airway, using NIS-Elements Basic Research software (Nikon, Tokyo, Japan).
Statistical Analysis
All results are presented as mean ± SEM from a minimum of three independent experiments. Group means were compared using the nonparametric Mann-Whitney U test (two-tailed). P < 0.05 was considered significant.
Results
FABP4 and FABP5 mRNA and Protein Expression in BPD
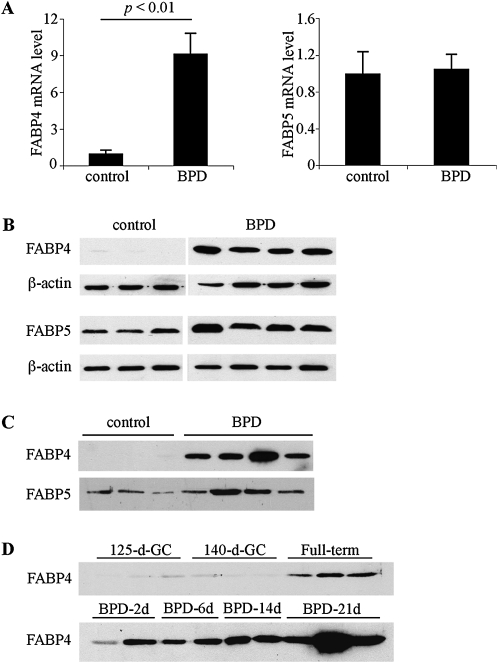
Whole lung relative mRNA expression levels of FABP4 and FABP5 were examined according to real-time PCR (Figure 1A). FABP4 mRNA levels demonstrated a significant increase in BPD tissues compared with gestational controls (GCs, P < 0.01), whereas FABP5 mRNA levels were similar in both groups. A comparison of lung FABP4 and FABP5 mRNA levels demonstrated that FABP5 mRNA levels were more than 100-fold and 10-fold higher than FABP4 mRNA levels in GC and BPD samples, respectively (not shown). Next, whole lung homogenates and necropsy bronchoalveolar lavage fluid (BALF) samples of GC and BPD group baboons were examined by immunoblotting and densitometry. FABP4 protein levels were very low in the lung homogenates of GC lungs, and were significantly increased in samples of BPD (P < 0.05, Figure 1B). Consistent with the mRNA results, FABP5 protein levels did not show a significant alteration in BPD (Figure 1B). Necropsy BALF samples from GC baboons did not reveal any FABP4 immunoreactivity, whereas strong FABP4 bands were detected in all BPD samples (Figure 1C). In contrast, FABP5 protein was detected in both GC and BPD samples at similar levels (Figure 1C, densitometry not shown). To examine whether FABP4 could be detected in BALF at earlier time points during the development of BPD, we analyzed necropsy BALF samples from BPD group baboons obtained 2, 6, 14, and 21 days after birth (Figure 1D). FABP4 levels in BALF increased with increasing days of BPD-inducing treatments in premature baboon lungs, starting on the second day of life. The highest levels were observed at the end of 21 days of treatment.
Figure 1.
The expression of fatty acid–binding protein 4 (FABP4) is increased in bronchopulmonary dysplasia (BPD). (A) Real-time RT-PCR for FABP4 and FABP5 was performed on total lung RNA, isolated from control and BPD group baboons (n = 6–7 samples per group). Relative expression levels were normalized to β-actin by the 2−ΔΔCT method. An arbitrary level of 1 was assigned to the control group. (B) FABP4 and FABP5 protein expression in whole baboon lung homogenates was analyzed by immunoblotting. β-actin was used as a loading control. (C) FABP4 and FABP5 were detected by immunoblotting in necropsy bronchoalveolar lavage fluid (BALF). (D) Time course of FABP4 detection in necropsy BALF samples.
Immunolocalization of FABP4 and FABP5 in BPD
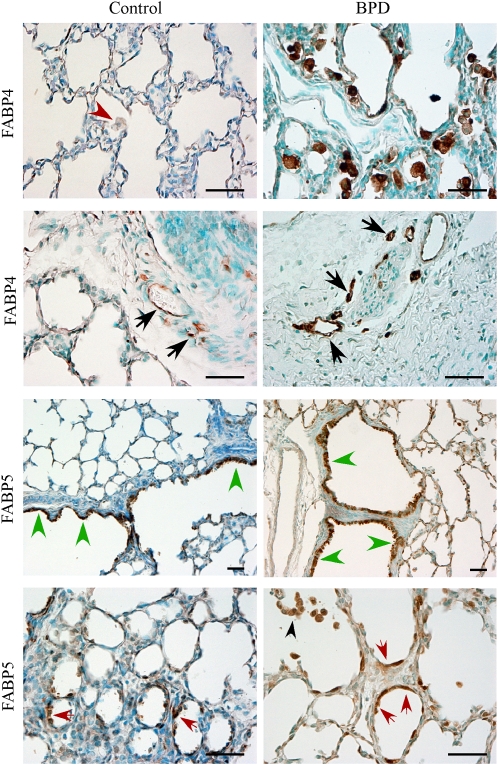
Immunohistochemistry was performed on paraffin-embedded baboon lung tissues to identify the cell types that expressed FABP4 and FABP5 in BPD (Figure 2). Control lung tissues contained very few macrophages, and these were FABP4-negative (Figure 2, red arrowhead), whereas strong FABP4 immunoreactivity was detected in the majority of alveolar macrophages in BPD samples. FABP4 was also detected in the endothelial cells of peribronchial vessels in both control and BPD lungs (Figure 2, black arrows). In contrast, no FABP4 immunoreactivity was evident in alveolar capillary endothelial cells in either the control or BPD tissues. In some samples of human BPD that also showed changes consistent with pulmonary hypertension, FABP4 was detected in vasa vasorum endothelial cells in the adventitia of pulmonary artery branches (Figures E1A and E1B in the online supplement). Some pleural blood vessels (Figures E1E and E1F) and lymphatics (Figure E1G and E1H) also contained FABP4-positive endothelial cells. This was evident in both BPD and control samples. In some samples of human BPD with severe fibrotic changes, FABP4 was also detected in microvascular ECs in fibrotic and subpleural areas (not shown).
Figure 2.
Immunolocalization of FABP4 and FABP5 in a baboon model of BPD. Representative images are shown. FABP4 was detected in macrophages in BPD, but not in control samples (first row, red arrowhead). FABP4 was also detected in peribronchial vascular endothelial cells in both control and BPD samples (second row, black arrowheads). FABP5 was localized to bronchiolar (third row, green arrowheads) and alveolar epithelial Type 2 cells (fourth row, red arrowheads), as well as macrophages (fourth row, black arrowhead). Scale bars = 50 μm.
Consistent with its expression pattern in mouse and rat lungs, FABP5 was detected in alveolar epithelial Type 2 cells in both GC and BPD samples (Figure 2, red arrows). In addition, strong FABP5 immunoreactivity was present in bronchiolar epithelial cells, and this immunoreactivity became weaker in bronchial epithelial cells. Macrophages also demonstrated FABP5 immunoreactivity in samples of BPD (Figure 2, black arrowhead). Occasional FABP5 immunoreactivity was detected in endothelial cells, including those in peribronchial areas.
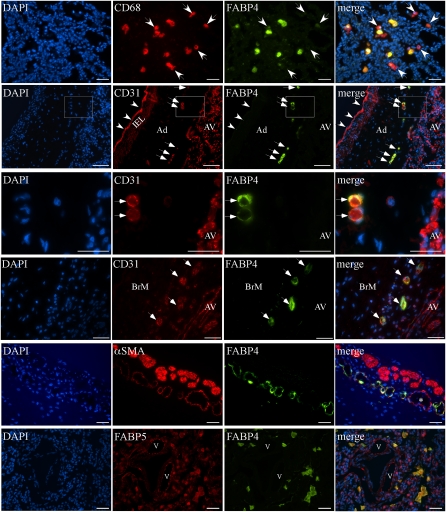
To characterize the expression patterns of FABP4 and FABP5 further, double immunofluorescence (IF) was performed on samples of baboon and human BPD. Representative images are shown in Figure 3. Double IF using antibodies against CD68 (a marker of macrophages) and FABP4 revealed that FABP4 was expressed in the majority, but not all, of CD68-positive macrophages (Figure 3, row 1). A similar pattern was evident after double IF using antibodies against CD163, another macrophage marker, and FABP4 (Figure E2E) (28). No preferential expression of FABP4 was evident in CD68 macrophage or CD163 macrophage subsets. Double IF with antibodies against CD31, a pan-EC marker, and FABP4 demonstrated colocalization in the majority of vascular ECs in peribronchial areas and in vasa vasorum ECs, whereas both microvascular and larger vascular ECs in alveolar areas expressed CD31 but not FABP4 (Figure 3, rows 2–4, and Figure E2). αSMA and FABP4 double IF revealed αSMA-expressing cells (pericytes or smooth muscle cells) around some, but not all, of the FABP4-positive peribronchial and perivascular microvessels (Figure 3, row 5, and Figure 2E). In general, an inverse correlation between FABP4 and αSMA immunoreactivity was evident: the vessels with the brightest FABP4 signal had weak or no αSMA expression. Double IF with FABP4 and FABP5 antibodies revealed diffuse FABP5 immunofluorescence, deriving from airway epithelial and pulmonary endothelial cells as well as macrophages in the lung parenchyma, whereas FABP4 was detected only in macrophages (Figure 3, row 6).
Figure 3.
Double immunofluorescence analysis for FABPs, CD31, CD68, and α-smooth muscle actin (αSMA) on baboon and human BPD sections. Representative images are shown. First row (baboon BPD): FABP4 is colocalized with CD68 in most, but not all, alveolar macrophages. White arrows indicate CD68-positive, but FABP4-negative, cells. Second row (human BPD): CD31 and FABP4 are colocalized in vasa vasorum ECs in the adventitia (Ad) of a pulmonary artery (white arrows), but not in pulmonary arterial ECs (white arrowheads) or alveolar vessels (AV). Internal elastic lamina (IEL) demonstrates autofluorescence. Third row: Higher magnification of the boxed area in row 2 demonstrates CD31 expression in alveolar vasculature and vasa vasorum ECs, but FABP4 expression only in vasa vasorum ECs (white arrows). Fourth row (human BPD): CD31 and FABP4 are colocalized in small vascular ECs in the bronchial mucosa (BrM, white arrows). Fifth row (human BPD): αSMA, a marker of mature pericytes and smooth muscle cells, is detected around some FABP4-positive peribronchial vessels. Larger vessels with continuous coverage of αSMA-expressing cells do not express FABP4 (asterisk). Sixth row (human BPD): In contrast to the widespread expression pattern of FABP5 in several cell types, including alveolar epithelial and endothelial cells lining pulmonary vessels (v) in the lung parenchyma, FABP4 is detected only in FABP5-positive alveolar macrophages. Scale bars = 25 μm.
FABP4-Positive Peribronchial Blood Vessels in Baboon and Human Samples of BPD
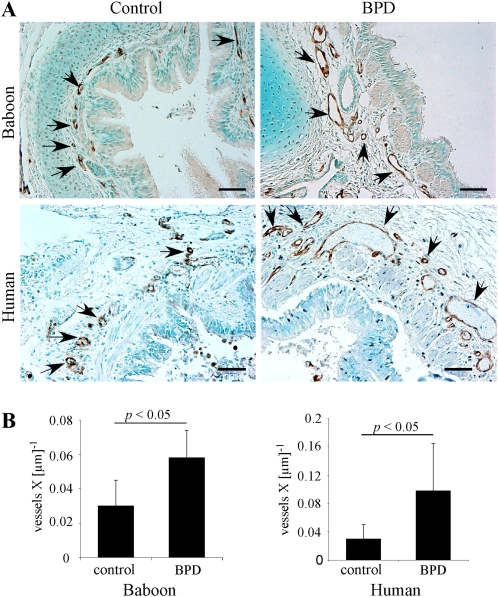
BPD is associated with the impaired capillary growth of lung parenchyma, which was postulated to underlie alveolar hypoplasia (29). However, the status of the bronchial vasculature in BPD was not examined previously, to the best of our knowledge. Our detailed characterization of FABP4 expression in baboon and human lungs indicated that FABP4 can be used reliably as a marker to differentiate between pulmonary and bronchial circulation–derived vasculature in the lung. Furthermore, the pattern of FABP4 immunostaining in the peribronchial vasculature suggested that a difference may exist in the density of peribronchial vessels between control and BPD samples. To confirm these initial observations, we quantified FABP4-positive peribronchial blood vessels, first using the baboon model of BPD, and then verified our findings in human samples. The patient characteristics are provided in Table 1. The gestational and chronologic ages at death were significantly greater in the control group (P < 0.01), but there was no difference in the postconceptional age at death. Quantitative analysis demonstrated a significantly higher number of FABP4-positive peribronchial vessels in both baboon and human lungs with BPD, compared with the GC samples (P < 0.05, Figure 4).
TABLE 1.
PATIENT CHARACTERISTICS
| Patient | Gestational Age (Weeks) | Chronologic Age at Death (Days) | Postconceptional Age at Death (Weeks) | Autopsy Diagnosis |
| Control | ||||
| 1 | 39 | 6 | 40 | HIE |
| 2 | 31 | 17 | 34 | NEC |
| 3 | 41 | 1 | 41 | HIE |
| 4 | 40 | 28 | 44 | SIDS |
| 5 | 40 | 60 | 49 | SIDS |
| Mean ± SD | 38.2 ± 4.0 | 22.4 ± 23.5 | 41.6 ± 5.5 | |
| BPD | ||||
| 1 | 24 | 150 | 46 | BPD, PHtn |
| 2 | 26 | 165 | 51 | BPD, PHtn |
| 3 | 24 | 59 | 32 | BPD |
| 4 | 28 | 90 | 41 | BPD, IUGR |
| 5 | 29 | 240 | 61 | BPD |
| Mean ± SD | 26.2 ± 2.3 | 140.8 ± 70.3 | 46.2 ± 10.8 | |
| P value* | <0.01 | <0.01 | NS |
Definition of abbreviations: BPD, bronchopulmonary dysplasia; HIE, hypoxic ischemic encephalopathy; IUGR, intrauterine growth restriction; NEC, necrotizing enterocolitis; NS, nonsignificant; PHtn, pulmonary hypertension; SIDS, sudden infant death syndrome.
Versus control group.
Figure 4.
FABP4-positive peribronchial blood vessel density is increased in BPD. (A) Representative images of immunohistochemistry for FABP4 demonstrate FABP4-positive peribronchial vessels (black arrowheads) in baboon and human lungs with and without BPD. Scale bars = 50 μm. (B) Peribronchial blood vessel density was quantitated and normalized to the airway perimeter in baboon and human infants with BPD (n = 5 samples per group).
Discussion
Inflammation plays a key role in the pathogenesis of BPD and in several other chronic lung diseases, such as chronic obstructive pulmonary disease and asthma. Although substantial evidence suggests a role for FABPs in regulating inflammation, this hypothesis has been addressed in only a limited number of studies involving lung disease (30). Here, we characterized the expression levels of FABP4 and FABP5 at the mRNA and protein levels, using a well-characterized baboon model of BPD, and found that FABP4 mRNA and protein concentrations in the lung were significantly higher than in control samples. FABP4 levels were also higher in necropsy BALF samples in the BPD group. In contrast, FABP5 concentrations did not demonstrate any significant alterations in either BALF or lung-tissue lysates. FABP4 was primarily expressed in macrophages and a subset of vascular endothelial cells, whereas FABP5 was detected in multiple cell types, including macrophages, epithelial cells, and endothelial cells. Interestingly, we noted a novel expansion of FABP4-positive peribronchial blood vessels in the baboon model of BPD, and confirmed this finding using samples of human BPD.
In a microarray study by Kompass and colleagues, FABP4 was also identified as one of the up-regulated genes in a sample of baboon BPD (31). The increased FABP4 concentrations in samples of BPD are likely attributable primarily to the macrophage influx in the airways, because we found macrophages to be the predominant cell type expressing FABP4 in BPD lungs. Interestingly, the expression of FABP4 was not uniform in all macrophages in samples of BPD. Using two different markers, CD68 and CD163, for M1-type and M2-type macrophages, respectively, we found that FABP4 was expressed in some, but not all, alveolar macrophages, and no preferential expression of FABP4 was evident in either of these macrophage subsets. Our observations, taken together with previous studies (15, 16, 32), suggest that FABP4 expression levels may correlate with the inflammatory activity of alveolar macrophages. Endothelial FABP4 may also have influenced the mRNA concentrations of FABP4 in our analysis, especially insofar as we used both proximal and distal lung samples for RNA isolation. In BALF samples, FABP4 was detected in significantly higher amounts in the BPD group. The mechanism by which FABP4 is released into BALF is not clear. However, several FABPs, including FABP4, were detected in urine and sera, and were elevated in various pathologic conditions (33, 34). Given the low molecular weight of FABPs (∼ 14 kD), leakage out of cells under certain circumstances, such as cell injury, may be one potential mechanism by which FABP4 is released into BALF. The findings of increased FABP4 concentrations in BALF as early as 2 days and the progressive increase through 21 days in the BPD group baboons suggest that airway FABP4 concentrations could serve as a biomarker of lung injury in BPD.
A role for FABP5 in surfactant synthesis was implicated, based on previous studies in murine and rat lungs. Recent studies also demonstrated an important role for FABP5 in the regulation of retinoic acid signaling (35). In the lung, FABP5 was detected at much higher concentrations compared with FABP4 because of its expression in multiple resident cell types, including bronchiolar and alveolar epithelial Type 2 cells. Based on this pattern of expression, we expected to find increased FABP5 concentrations in BPD, because BPD is associated with alveolar epithelial Type 2 cell hyperplasia. However, this was not the case. In contrast with FABP4, FABP5 concentrations remained steady in both lung tissue and BALF samples with BPD.
Studies have shown decreased concentrations of vascular endothelial growth factor (VEGF)–A in baboon and human lungs with BPD, in association with dysmorphic alveolar microvessels (36, 37). The importance of disrupted VEGF signaling and the impaired growth of alveolar capillaries in BPD are also supported by studies of rodent models, where the exogenous supplementation of VEGF can rescue not only capillary growth, but also alveolarization in the lung (38–40). Interestingly, the spectrum of dysmorphic vasculature in BPD includes the up-regulation of some vascular endothelial cell–specific molecules, such as endoglin (41, 42). Thus far, BPD-related vascular research has focused primarily on alveolar capillaries, which arise from the pulmonary vascular bed. The lung also receives a blood supply from the bronchial circulation, which primarily perfuses the peribronchial areas, the visceral pleura, and the vasa vasorum (43, 44). In this study, we found that FABP4 was expressed in endothelial cells in all these areas, but not in the pulmonary vasculature, thus confirming its specificity for the first time as a bronchial circulation–specific marker.
Although the bronchial circulation is known to be far more angiogenic than the pulmonary circulation, the mechanisms underlying this difference remain poorly understood (43, 45). Furthermore, the status of bronchial circulation–derived vessels in BPD was not, to the best of our knowledge, investigated previously. We took advantage of FABP4 as a differentially expressed marker of the bronchial vasculature, and examined the density of peribronchial vessels in samples of baboon and human BPD. This analysis demonstrated for the first time that BPD is associated with an expansion of peribronchial blood vessels.
Several important questions and implications relevant to the pathogenesis of BPD were raised by our observations. First, the two vascular beds in the lung appear to give distinct and opposing responses to the injury that occurs in BPD, with the bronchial vasculature eliciting an angiogenic response, in contrast to the impaired angiogenic response of the pulmonary microvasculature. Second, the expansion of the peribronchial vasculature in BPD may represent pathologic rather than physiologic angiogenesis, and may contribute to airway obstruction in BPD (46) by causing increased vessel leakiness and mucosal edema. The lack of αSMA-positive cells around some FABP4-positive vessels supports this concept. Similar hypotheses were proposed regarding the functional consequences of increased peribronchial vessel density in asthmatic airways (45, 47, 48). Interestingly, infants with BPD are at higher risk for developing asthma-like symptoms in later life (49), and peribronchial pathological angiogenesis could serve as a link between these two diseases.
Another intriguing question raised by our findings is whether FABP4 is simply a marker of the bronchial microvasculature, or a mediator that contributes to the regulation of the angiogenic activity of bronchial vascular ECs. We recently reported that FABP4 is strongly induced by VEGF-A via VEGF receptor 2, and showed that it promotes the proliferation of human umbilical vein and dermal microvascular endothelial cells in vitro (14). Consistent with our data, FABP4 was identified as the most up-regulated EC gene, with an impressive, approximately 100-fold induction in a study that examined gene expression patterns associated with neoangiogenesis in a wound-healing model (50). These findings, taken together with our present observations, strongly suggest that FABP4 may play a novel role in enhancing the angiogenic capacity of the bronchial microvasculature. Consistent with this role, lack of FABP4 expression in alveolar capillary ECs may account, at least in part, for the relatively suppressed angiogenic response of these cells, as occurs in BPD. Studies are underway to address this hypothesis as well as the role of FABP4 in the inflammatory activity of macrophages in BPD.
Supplementary Material
Acknowledgments
The authors thank Dr. Mary Williams and Dr. Alan Fine for helpful discussions.
Footnotes
Part of this manuscript was presented in 2009 at the Pediatric Academic Society Meetings in Baltimore, Maryland.
This study was supported by the Peabody Foundation (S.C.), the Brigham and Women's Hospital Biomedical Research Institute (S.C.), and National Institutes of Health grant HL075904 (S.C.).
Cagatay Karaaslan is presently at the Department of Biology, Hacettepe University, Ankara, Turkey.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0376OC on December 22, 2010
Author Disclosure: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, et al. 2007. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol 2007;147.e1–8 [DOI] [PubMed] [Google Scholar]
- 2.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 2007;357:1946–1955 [DOI] [PubMed] [Google Scholar]
- 3.Eichenwald EC, Stark AR. Management and outcomes of very low birth weight. N Engl J Med 2008;358:1700–1711 [DOI] [PubMed] [Google Scholar]
- 4.Kallapur SG, Jobe AH. Contribution of inflammation to lung injury and development. Arch Dis Child Fetal Neonatal Ed 2006;91:F132–F135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bancalari E, Claure N. Non-invasive ventilation of the preterm infant. Early Hum Dev 2008;84:815–819 [DOI] [PubMed] [Google Scholar]
- 6.Doyle LW, Faber B, Callanan C, Freezer N, Ford GW, Davis NM. Bronchopulmonary dysplasia in very low birth weight subjects and lung function in late adolescence. Pediatrics 2006;118:108–113 [DOI] [PubMed] [Google Scholar]
- 7.Wang LY, Luo HJ, Hsieh WS, Hsu CH, Hsu HC, Chen PS, Chiu NC, Lee WT, Jeng SF. Severity of bronchopulmonary dysplasia and increased risk of feeding desaturation and growth delay in very low birth weight preterm infants. Pediatr Pulmonol 2010;45:165–173 [DOI] [PubMed] [Google Scholar]
- 8.Gagliardi L, Bellu R, Zanini R, Dammann O. Network Neonatale Lombardo Study Group. Bronchopulmonary dysplasia and brain white matter damage in the preterm infant: a complex relationship. Paediatr Perinat Epidemiol 2009;23:582–590 [DOI] [PubMed] [Google Scholar]
- 9.Short EJ, Klein NK, Lewis BA, Fulton S, Eisengart S, Kercsmar C, Baley J, Singer LT. Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8-year-old outcomes. Pediatrics 2003;112:e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hertzel AV, Bernlohr DA. The mammalian fatty acid–binding protein multigene family: molecular and genetic insights into function. Trends Endocrinol Metab 2000;11:175–180 [DOI] [PubMed] [Google Scholar]
- 11.Haunerland NH, Spener F. Fatty acid–binding proteins: insights from genetic manipulations. Prog Lipid Res 2004;43:328–349 [DOI] [PubMed] [Google Scholar]
- 12.Zimmerman AW, Veerkamp JH. New insights into the structure and function of fatty acid–binding proteins. Cell Mol Life Sci 2002;59:1096–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makowski L, Hotamisligil GS. The role of fatty acid binding proteins in metabolic syndrome and atherosclerosis. Curr Opin Lipidol 2005;16:543–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elmasri H, Karaaslan C, Teper Y, Ghelfi E, Weng M, Ince TA, Kozakewich H, Bischoff J, Cataltepe S. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J 2009;23:3865–3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS. The fatty acid–binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity: macrophage expression of aP2 impacts peroxisome proliferator–activated receptor gamma and IkappaB kinase activities. J Biol Chem 2005;280:12888–12895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds JM, Liu Q, Brittingham KC, Liu Y, Gruenthal M, Gorgun CZ, Hotamisligil GS, Stout RD, Suttles J. Deficiency of fatty acid–binding proteins in mice confers protection from development of experimental autoimmune encephalomyelitis. J Immunol 2007;179:313–321 [DOI] [PubMed] [Google Scholar]
- 17.Jonsson B, Tullus K, Brauner A, Lu Y, Noack G. Early increase of TNF alpha and IL-6 in tracheobronchial aspirate fluid indicator of subsequent chronic lung disease in preterm infants. Arch Dis Child Fetal Neonatal Ed 1997;77:F198–F201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotecha S, Wilson L, Wangoo A, Silverman M, Shaw RJ. Increase in interleukin (IL)–1 beta and IL-6 in bronchoalveolar lavage fluid obtained from infants with chronic lung disease of prematurity. Pediatr Res 1996;40:250–256 [DOI] [PubMed] [Google Scholar]
- 19.Bourbia A, Cruz MA, Rozycki HJ. NF-kappaB in tracheal lavage fluid from intubated premature infants: association with inflammation, oxygen, and outcome. Arch Dis Child Fetal Neonatal Ed 2006;91:F36–F39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aghai ZH, Kode A, Saslow JG, Nakhla T, Farhath S, Stahl GE, Eydelman R, Strande L, Leone P, Rahman I. Azithromycin suppresses activation of nuclear factor–kappa B and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatr Res 2007;62:483–488 [DOI] [PubMed] [Google Scholar]
- 21.Rehan VK, Wang Y, Patel S, Santos J, Torday JS. Rosiglitazone, a peroxisome proliferator–activated receptor–gamma agonist, prevents hyperoxia-induced neonatal rat lung injury in vivo. Pediatr Pulmonol 2006;41:558–569 [DOI] [PubMed] [Google Scholar]
- 22.Guthmann F, Hohoff C, Fechner H, Humbert P, Borchers T, Spener F, Rustow B. Expression of fatty-acid–binding proteins in cells involved in lung-specific lipid metabolism. Eur J Biochem 1998;253:430–436 [DOI] [PubMed] [Google Scholar]
- 23.Owada Y, Abdelwahab SA, Suzuki R, Iwasa H, Sakagami H, Spener F, Kondo H. Localization of epidermal-type fatty acid binding protein in alveolar macrophages and some alveolar Type II epithelial cells in mouse lung. Histochem J 2001;33:453–457 [DOI] [PubMed] [Google Scholar]
- 24.Guthmann F, Schachtrup C, Tolle A, Wissel H, Binas B, Kondo H, Owada Y, Spener F, Rustow B. Phenotype of palmitic acid transport and of signalling in alveolar Type II cells from E/H-FABP double-knockout mice: contribution of caveolin-1 and PPARgamma. Biochim Biophys Acta 2004;1636:196–204 [DOI] [PubMed] [Google Scholar]
- 25.Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med 1999;160:1333–1346 [DOI] [PubMed] [Google Scholar]
- 26.Altiok O, Yasumatsu R, Bingol-Karakoc G, Riese RJ, Stahlman MT, Dwyer W, Pierce RA, Bromme D, Weber E, Cataltepe S. Imbalance between cysteine proteases and inhibitors in a baboon model of bronchopulmonary dysplasia. Am J Respir Crit Care Med 2006;173:318–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasumatsu R, Altiok O, Benarafa C, Yasumatsu C, Bingol-Karakoc G, Remold-O'Donnell E, Cataltepe S. SERPINB1 upregulation is associated with in vivo complex formation with neutrophil elastase and cathepsin G in a baboon model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2006;291:L619–L627 [DOI] [PubMed] [Google Scholar]
- 28.Fabriek BO, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor CD163. Immunobiology 2005;210:153–160 [DOI] [PubMed] [Google Scholar]
- 29.Abman SH. Bronchopulmonary dysplasia: a vascular hypothesis. Am J Respir Crit Care Med 2001;164:1755–1756 [DOI] [PubMed] [Google Scholar]
- 30.Shum BO, Mackay CR, Gorgun CZ, Frost MJ, Kumar RK, Hotamisligil GS, Rolph MS. The adipocyte fatty acid–binding protein aP2 is required in allergic airway inflammation. J Clin Invest 2006;116:2183–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kompass KS, Deslee G, Moore CH, McCurnin D, Pierce RA. Highly conserved transcriptional responses to mechanical ventilation of the lung. Physiol Genomics 2010;42:384–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hui X, Li H, Zhou Z, Lam KS, Xiao Y, Wu D, Ding K, Wang Y, Vanhoutte PM, Xu A. Adipocyte fatty acid binding protein mediates inflammatory responses in macrophages through a positive feedback loop involving c-Jun N-terminal kinases and activator protein-1. J Biol Chem 2010;285:10273–10280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cabre A, Lazaro I, Girona J, Manzanares JM, Marimon F, Plana N, Heras M, Masana L. Fatty acid binding protein 4 is increased in metabolic syndrome and with thiazolidinedione treatment in diabetic patients. Atherosclerosis 2007;195:e150–e158 [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama T, Kamijo-Ikemori A, Sugaya T, Hoshino S, Yasuda T, Kimura K. Urinary excretion of liver type fatty acid binding protein accurately reflects the degree of tubulointerstitial damage. Am J Pathol 2009;174:2096–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell 2007;129:723–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H. Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol 2002;282:L811–L823 [DOI] [PubMed] [Google Scholar]
- 37.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;164:1971–1980 [DOI] [PubMed] [Google Scholar]
- 38.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 2000;279:L600–L607 [DOI] [PubMed] [Google Scholar]
- 39.Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation 2005;112:2477–2486 [DOI] [PubMed] [Google Scholar]
- 40.Kunig AM, Balasubramaniam V, Markham NE, Morgan D, Montgomery G, Grover TR, Abman SH. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am J Physiol Lung Cell Mol Physiol 2005;289:L529–L535 [DOI] [PubMed] [Google Scholar]
- 41.De Paepe ME, Patel C, Tsai A, Gundavarapu S, Mao Q. Endoglin (CD105) up-regulation in pulmonary microvasculature of ventilated preterm infants. Am J Respir Crit Care Med 2008;178:180–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abman SH. The dysmorphic pulmonary circulation in bronchopulmonary dysplasia: a growing story. Am J Respir Crit Care Med 2008;178:114–115 [DOI] [PubMed] [Google Scholar]
- 43.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res 2007;100:174–190 [DOI] [PubMed] [Google Scholar]
- 44.Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol 2005;67:623–661 [DOI] [PubMed] [Google Scholar]
- 45.Charan NB, Baile EM, Pare PD. Bronchial vascular congestion and angiogenesis. Eur Respir J 1997;10:1173–1180 [DOI] [PubMed] [Google Scholar]
- 46.Lum S, Kirkby J, Welsh L, Marlow N, Hennessy E, Stocks J. Nature and severity of lung function abnormalities in extremely preterm children at 11 y. Eur Respir J 2010 Oct 14. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Salvato G. Quantitative and morphological analysis of the vascular bed in bronchial biopsy specimens from asthmatic and non-asthmatic subjects. Thorax 2001;56:902–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hashimoto M, Tanaka H, Abe S. Quantitative analysis of bronchial wall vascularity in the medium and small airways of patients with asthma and COPD. Chest 2005;127:965–972 [DOI] [PubMed] [Google Scholar]
- 49.Greenough A. Late respiratory outcomes after preterm birth. Early Hum Dev 2007;83:785–788 [DOI] [PubMed] [Google Scholar]
- 50.Soulet F, Kilarski WW, Antczak P, Herbert J, Bicknell R, Falciani F, Bikfalvi A. Gene signatures in wound tissue as evidenced by molecular profiling in the chick embryo model. BMC Genomics 2010 Sep 14;11:495 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.