Figure 2. Protein import into mitochondria.
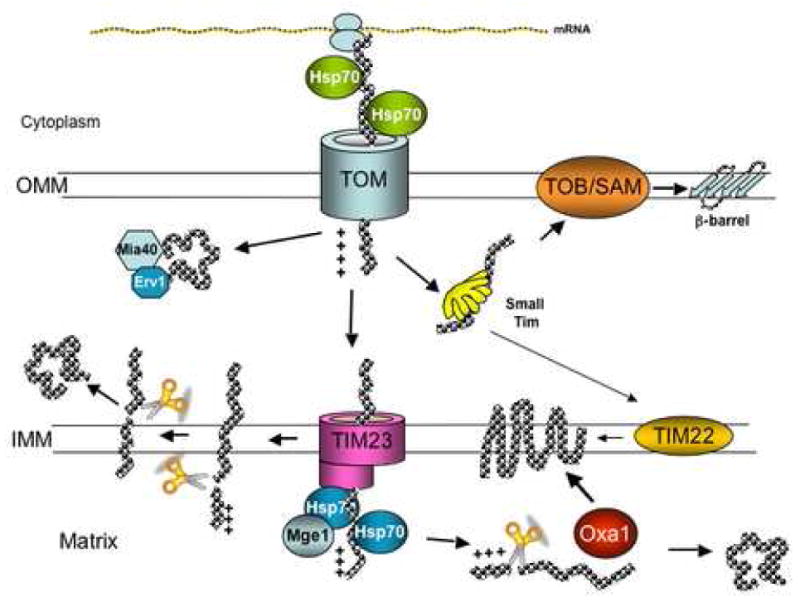
Nuclear encoded proteins are imported into mitochondria by different pathways depending on their final destination. Matrix proteins, synthesized as precursors containing a targeting sequence in the amino terminus are translocated through TOM and TIM23-Hsp70/Mge1 complexes and once in the matrix the targeting sequence is cleaved by mitochondrial proteases to render the mature form. Inner membrane proteins are translocated by TOM and TIM23 but get arrested in the latter, transferred laterally into the membrane and have their targeting sequence cleaved. Other inner membrane proteins are imported into the matrix by TOM and TIM23 and inserted into the inner membrane with the help of Oxa1. In addition, very hydrophobic inner membrane proteins with even number of transmembrane domains are translocated through TOM, then passed to TIM22 via small Tim proteins and inserted into the membrane. Intermembrane space (IMS) proteins are transported via TOM-TIM23 and laterally transferred to inner membrane, where the presequence is cleaved and the protein is released into the intermembrane space. Other IMS proteins are translocated through the TOM complex and then folded in the intermembrane space with the help of Mia40 and Erv1 chaperones. Outer membrane proteins are translocated through the TOM complex, guided by the small Tim proteins to the TOB/SAM complex and inserted into the membrane. See text for more details.
