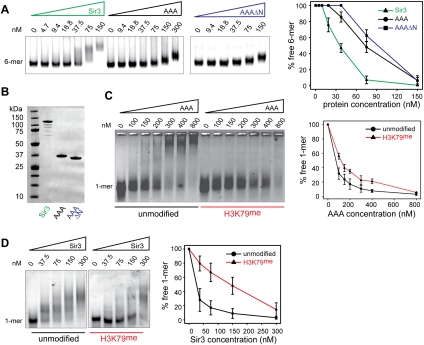
Figure 3.
Binding of the Sir3 AAA+ ATPase-like domain to chromatin was sensitive to methylation of H3K79. (A) The Sir3 protein, the Sir3 AAA+ ATPase-like domain (AAA; amino acids 530–845), or an N-terminal truncation (AAAΔN; amino acids 545–845) was titrated over a constant amount (25 nM) of unmodified 6-mer nucleosomes. (B) SDS-PAGE gel of 1 μg of the Sir3 protein, the Sir3 AAA+ domain, and the N-terminal truncation used in the experiments above (staining with Coomassie brilliant blue). (C) The Sir3 AAA+ ATPase-like domain was titrated over a constant amount (25 nM) of unmodified or H3K79me Cy3-147 mononucleosomes. Samples were separated by native agarose gel electrophoresis, and Cy3-labeled DNA was visualized. The images are representative of at least three independent experiments, and quantifications show the mean value ± SEM of the percent of unbound chromatin compared with the input. (D) Full-length Sir3 was titrated over a constant amount (25 nM) of unmodified or H3K79me mononucleosomes; three independent experiments were analyzed and plotted as described in C.