This study demonstrates that sPLA2-IIa significantly amplifies inflammation of a compromised ocular surface, which can be reversed by its specific inhibitor S-3319. This mechanism could lead to a novel therapeutic intervention for ocular surface inflammatory disorders, such as DE disease.
Abstract
Purpose.
sPLA2-IIa is a biomarker for many inflammatory diseases in humans and is found at high levels in human tears. However, its role in ocular surface inflammation remains unclear. An experimentally induced BALB/c mouse dry eye (DE) model was used to elucidate the role of sPLA2-IIa in ocular surface inflammation.
Methods.
BALB/c mice were subcutaneously injected with scopolamine and placed in a daytime air-drying device for 5 to 10 days. Control mice received no treatment. DE status was evaluated with tear production with a phenol-red thread method. Tear inflammatory cytokines were quantified by multiplex immunoassays. Ocular surface inflammation and sPLA2-IIa expression were examined by immune-staining and quantitative (q)RT2-PCR. Conjunctiva (CNJ) of the mice was cultured for prostaglandin E2 production induced by sPLA2-IIa with various amount of sPLA2-IIa inhibitor, S-3319.
Results.
Treated mice produced fewer tears and heavier corneal (CN) fluorescein staining than the untreated controls (P < 0.001). They also revealed lower goblet cell density (P < 0.001) with greater inflammatory cell infiltration within the conjunctiva, and higher concentration of tear inflammatory cytokines than the controls. Moreover, treated mice showed heavier sPLA2-IIa immune staining than the controls in the CNJ epithelium, but not in the CN epithelium or the lacrimal gland. Treated mice exhibited upregulated sPLA2-IIa and cytokine gene transcription. Furthermore, CNJ cultures treated with sPLA2-IIa inhibitor showed significantly reduced sPLA2-IIa-induced inflammation.
Conclusions.
This is the first report regarding sPLA2-IIa in the regulation of ocular surface inflammation. The findings may therefore lead to new therapeutic strategies for ocular surface inflammation, such as DE disease.
Phospholipase A2 (PLA2) is a superfamily of enzymes that hydrolyze glycerophospholipids through the “sn-2” position to produce lysophospholipids and free fatty acids (FFAs). The FFAs produced include arachidonic acid, which is the precursor of well-known inflammatory mediators, such as PGE2, leukotrienes, and eicosaniods.1 At least five types of PLA2 have been reported in humans and rodents according to their functional locations or calcium requirements: secretory PLA2 (sPLA2), cytosolic PLA2 (cPLA2), calcium-independent PLA2 (iPLA2), platelet-activating factor acetylhydrolase (PAF-AH), and lysosomal PLA2 (LPLA2).2,3 A new type of PLA2, a calcium-dependent adipose-specific PLA2 (AdPLA2), was recently added to the list as a major regulator of adipocyte lipolysis crucial in the development of obesity.4
Humans and rodents share at least 10 isoforms of sPLA2.5 Of these, the human group IIc isoform (sPLA2-IIc) is a nonfunctional pseudogene.2 At least three isoforms, sPLA2-IIa, sPLA2-V, and sPLA2-X, have been shown to be associated with human inflammatory diseases such as atherosclerosis and rheumatoid arthritis.6–8 Group IIa sPLA2 (sPLA2-IIa) is the prototype of the sPLA2 family and one of the most intensely studied isoforms. The association of elevated sPLA2-IIa levels with the inflammatory process has been shown in a number of human diseases, including rheumatoid arthritis,6 atherosclerosis,9 inflammatory bowel disease,10 septic shock,11 and asthma.12 For example, both the concentration and activity of sPLA2-IIa were shown to be increased in the synovial fluid of rheumatoid arthritis patients. A recent study of sPLA2s13 using BALB/c and C57BL/6 transgenic mice confirmed the contributions of human sPLA2-IIa to the severity of arthritis in a K/BxN serum-transferring mouse model. The same study also revealed a novel anti-inflammatory role of sPLA2-V, a closely related isoform to sPLA2-IIa, in the pathogenesis of the arthritis model. Systematic studies on the expression and distribution of sPLA2 isoforms, especially for groups IIa, IIe, and X, revealed an important role of sPLA2-IIa in oligodendrocyte death in the central nervous system compromised by H2O2, IL-1β, and TNF-α.14 sPLA2-IIa is therefore considered one of the most important biomarkers for these inflammatory diseases. Furthermore, the noncatalytic functions of sPLA2-IIa were shown to be important in inflammation and cancer cell signaling.15–18
It has been reported that the level of sPLA2-IIa in normal human tears is at least 10,000 times higher than that in normal human serum.19 sPLA2-IIa is considered a major component of the eye's innate immune defense system against Gram-positive bacteria.20 The human sPLA2-IIa gene (PLA2G2A) was shown to be expressed approximately 6500 times more in human conjunctiva (CNJ) compared with the human cornea (CN), representing the greatest difference in gene expression between the CNJ and CN.21 It was documented that both the concentration and enzymatic activity of sPLA2-IIa were elevated twofold in the tears of dry eye (DE) disease patients when compared to the tears of age-matched normal controls.19,22 Furthermore, sPLA2-IIa stimulated PGE2 production in a proinflammatory cytokine-dependent manner in human CNJ epithelial cell line cultures and mouse CNJ organ cultures,22 indicating a role of sPLA2-IIa as an inflammatory mediator on the compromised ocular surface. However, the molecular mechanism of sPLA2-IIa with respect to ocular surface inflammation, beyond its bactericidal effect, remains unclear.
DE disease is suggested as an inflammation of the ocular surface, and it affects the quality of daily life of millions of people.23 It more commonly affects women, especially in the aged population. Studies have demonstrated that chronic DE diseases often involve comprehensive interactions among immune cells such as T lymphocytes, dendritic cells, macrophages, and epithelia that cause the release of various types of cytokines, chemokines, and matrix metalloproteinases.24–29 Several mouse DE models have been established using C57BL/6 and BALB/c mice to study these immune interactions. DE is induced in these mice by either subcutaneous (SC) scopolamine injection, air ventilation, or both.30–32 In this study, we used DE disease as an ocular surface inflammatory disease model and adopted the scopolamine-air ventilation DE device to induce DE in mice. Due to the lack of a functional sPLA2-IIa gene in C57BL/6 (pla2g2a−),2 we chose BALB/c mice, which have a functional sPLA2-IIa gene, for our mouse work on sPLA2-IIa. This article shows that the tear secretion deficiency in the experimental DE BALB/c mice, induced by the scopolamine-air ventilation method, is accompanied by elevated sPLA2-IIa levels and ocular surface inflammation. We further demonstrate, by using a specific sPLA2-IIa inhibitor (S-3319), that sPLA2-IIa plays an important role in amplifying ocular surface inflammation.
Materials and Methods
Mouse DE Model
The protocol for the mouse DE model was approved by the Institutional Animal Care and Use Committee of Mount Sinai School of Medicine, complying with the standards in the Association for Research in Vision and Ophthalmology Statement for the Use of Animal in Ophthalmic and Vision Research. The published C57BL/6 mouse DE models (scopolamine injection-ventilation fan system) were adopted for in-house BALB/c mice experiments.30,31,33–36 Mice were assayed in four independent experiments using at least 20 BALB/c mice each (6–8 weeks old). In each experiment, half of the mice (equally split into two groups) were injected SC with 200 μL of 2.5 mg/mL scopolamine 4 times a day (9 AM, 12 PM, 3 PM, and 6 PM) from day 1 to day 10. The injected mice were placed in a specially designed mouse cage with a fan and placed under a negatively circulated air hood to remove humidity during the day. The other half of the mice in each experiment (also split equally into two groups) served as controls; they did not receive scopolamine injection and were kept under normal environmental conditions. Per mouse, one eye was used for histologic analyses and the other eye was used for qRT2-PCR.
Tear Thread
At days “0–1,” “5,” and “10” posttreatment initiation, tear production in both eyes of each mouse was measured using phenol-red cotton thread (Zone-Quick; Lacrimedics, Eastsound, WA). The thread was held with a pair of forceps and placed in the lateral canthus of the CNJ fornix for 30 seconds, and the length of the wetted cotton thread was recorded. Pooled threads from each experimental group or two eyes of each mouse were stored immediately at −80°C for later use in cytokine assay. Immediately before initiating the cytokine assay, the pooled threads were incubated with 60 μL of the assay buffer at room temperature for 1 hour and spun down twice at 10,000 rpm for 10 minutes at 4°C. This clarified liquid was then used for the assay.
CN Staining
CN fluorescein staining was performed and recorded by an experienced ophthalmologist using standard procedures. In brief, 1 μL of 5% sodium fluorescein solution in PBS was instilled into the inferior CNJ sac of each eye for 5 minutes. The CN staining was then recorded by a digital camera using slit-lamp microscopy in cobalt blue light, and corneal keratopathy was scored with a standard (National Eye Institute) grading system37–40that adds up a 0–3 score from each of the five divided areas in corneas.
Immunoassays of Tear Cytokines
Tear cytokines were measured using pooled tear thread32 with a mouse cytokine/chemokine kit from Millipore (MultiPlex, MPXMCYTO-70K; Millipore Corp., St. Charles, MO) on a system (Luminex 100; Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. Standard curves were generated by using the reference cytokine concentrations supplied by the manufacturer. Raw data (mean fluorescent intensity) were analyzed by commercially available software (Bio-Plex Manager Software; Bio-Rad Laboratories) and converted into concentration values.
Hematoxylin-Eosin and Periodic Acid Schiff Stain
The superior CNJ of euthanatized mice was marked with black ink to reference of orientation. The entire eyeball, including the eyelids, was surgically enucleated, fixed in PBS-buffered 10% formalin at 4°C overnight, and embedded in paraffin. A 6 μm-thick cross section through the central vertical plane was stained with hematoxylin-eosin and periodic acid-Schiff reagent according to standard protocols. The morphology of the CNJ and CN epithelium was assessed under a bright-field microscope (Zeiss Axioplan 2IE; Carl Zeiss, Thornwood, NY, in Mount Sinai School of Medicine Shared Resource Facilities), by two independent unbiased observers and recorded by digital camera. Goblet cells and infiltrated inflammatory cells in the superior and inferior bulbar and tarsal CNJ were counted by an unbiased observer using commercially available software (Photoshop CS4; Adobe Systems, Inc., San Jose, CA; and ImageJ, http://rsbweb.nih.gov/ij/docs/index.html). At least three different sections of each specimen were counted; the averages and standard deviations of the three were then used for analysis.
Immunofluorescence Staining
The entire eyeball from BALB/c or C57BL/6 mice, including the eyelids, was surgically enucleated, immediately embedded in optimal cutting temperature compound, flash-frozen in liquid nitrogen, and stored at −80°C. The cryosections (6 μm thick) were mounted on slides (Color Frost/Plus, no. 12-550-18; Fisher Scientific, Suwanee, GA), air dried for 1 hour, fixed by 100% acetone, and blocked with PBS-10% normal donkey serum (no. 000-001, Jackson ImmunoResearch Laboratories). The slides were then stained with polyclonal goat anti-mouse sPLA2-IIa IgG (no. sc-I4472; Santa Cruz Biotechnology, Santa Cruz, CA), followed by polyclonal donkey anti-goat IgG (Alexa Fluor-488 or Alexa Fluor-594–labeled, nos. A11055, A11058; Jackson ImmunoResearch Laboratories, Invitrogen CO, Grand Island, NY) and 4′-6-diamidino-2-phenylindole. Fluorescent signals were visualized on a fluorescence microscope (Zeiss Axioplan 2IE; Carl Zeiss, in Mount Sinai School of Medicine Shared Resource Facilities). Digital photos were taken to cover the entire CNJ region of each eye sample. The sPLA2-IIa+ cells were then counted using commercially available software (Photoshop CS4, Adobe Systems, Inc.; and ImageJ, http://rsbweb.nih.gov/ij/docs/index.html) by an unbiased observer.
Quantitative Real-Time RT-PCR (qRT2-PCR)
The CNJ tissues of euthanatized mice were removed under a dissecting microscope while the CN epithelial cells were obtained by scratching with a razor blade. Both tissues were immediately placed into tubes containing Trizol reagent; RNA was isolated using the Trizol method, followed by DNA digestion, RNA purification, cDNA synthesis, and quantitative real-time RT-PCR (qRT2-PCR). A set of established primers from QIAGEN (Valencia, CA) was used for qRT2-PCR: sPLA2-IIa (QT00109977), IFN-γ (QT01038821), IL-6 (QT00098875), IL-17A (QT00103278), IL-17F (QT00144347), and IL-1β (QT01048355). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (QT01658692) was used as a control for normalization.
CNJ Organ Cultures and PGE2 Production
The CNJ organs of euthanatized mice were surgically removed, washed with ice-cold PBS, and seeded into a 96-well plate containing 200 μL of Shem's medium supplemented with 5% fetal bovine serum. After one hour preincubation in 95% air and 5% CO2 at 37°C, the CNJ organs were treated for 12 hours with fresh Shem's medium containing either 25 μg/mL of human recombinant sPLA2-IIa (no. 5374-PL), 10 ng/mL of TNF-α (no. 210-TA), and 10 ng/mL of IL-1β (no. 201-LB) or sPLA2-IIa and TNF-α or sPLA2-IIa and IL-1β. All reagents are from R&D Systems (Minneapolis, MN). After treatment, the medium was analyzed for PGE2 production according to the manufacturer's instructions (EIA, no. 514,010; Cayman Chemical, Ann Arbor, MI).
Statistical Analysis
A sample size of 20 eyes was calculated to have approximately a 92% power of detecting between treated and untreated groups via a POWER analysis data simulation. Data were first averaged between samples within an experiment and then between experiments, and results were presented as the mean ± SD. These parametric data were analyzed for statistical differences, using the mean ± SD for each of the various groups in computer generated two-tailed bivariant Student's t-tests (NCSS; NCSS Inc., Kaysville, UT; SPSS; SPSS Inc., Chicago, IL), as well as a one-way analysis of variance (ANOVA: NCSS, SPSS). Two-tailed significance was established at a confidence level of 0.05 > P > 0.95.
Results
The Ocular Surface of Scopolamine-Treated Mice Was Dried, Damaged, and Inflamed
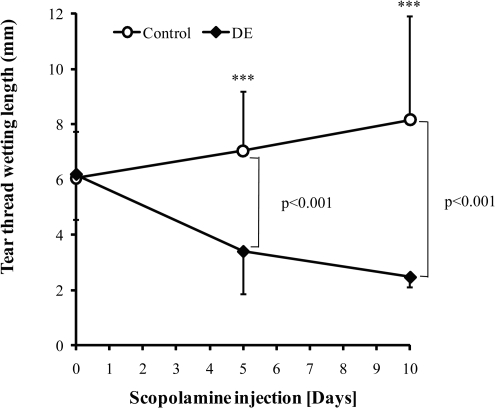
Scopolamine injection with air ventilation has been shown to be an effective method to induce DE disease in mice.41,42 To study the role of sPLA2-IIa in DE pathogenesis, we first adopted the published methods and induced DE disease in BALB/c mice. As shown in Figure 1, the basal level of tear production for each group was measured with the phenol-red thread test. At day “0” (before treatment began), no differences were found among groups with respect to tear production. After 5 days of scopolamine-air ventilation treatment, the treated mice showed significant reduction (P < 0.001) in tear production compared with that of the untreated control mice. This trend of reduction in tear production continued over the 10-day treatment period (Fig. 1), suggesting that the ocular surface was dried as intended.
Figure 1.
Tear production measurement with phenol-red cotton thread method. Equal numbers of BALB/c mice (6–8 weeks) were injected with 1 mg of scopolamine per day (0.25 mg in 200 μL of sterilized distilled water for 4 times per day, in 3-hour intervals) and placed in a daytime air-drying device for 5–10 days. Control mice received no treatment. Tear thread wetting length was measured for both eyes of each mouse at days “0,” “5,” and “10.” Student's t-test was used for statistical analysis. Data are the mean ± SD from four independent experiments. A total of 65 mice were used.
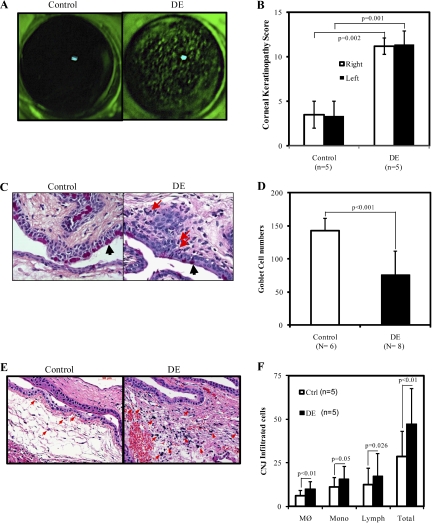
To determine whether or not these dried ocular surfaces caused any damage to the ocular surface, the surface integrity of the CN was examined by experienced ophthalmologists using the CN fluorescein staining method. As shown in Figure 2A, the CN surface of DE mice showed significant dense fluorescein staining with the typical superficially punctuated pattern (Fig. 2A, right panel), indicating damage of the dried CN surface; the CN surface of control mice (Fig. 2A, left panel) appeared clear with no to minimal staining. The corneal keratopathy scores of DE mice were then recorded and shown to be significantly higher than those of the control mice (P = 0.002 for the right eyes, and P = 0.001 for the left). The scores of both eyes from each animal showed no differences (Fig. 2B).
Figure 2.
The pathologic changes of mouse eyes. (A) Corneal (CN) fluorescein staining of control (left) or scopolamine-treated DE (right) mice. (B) Corneal keratopathy scores of control (left) or DE mice (right). (C) Periodic acid Schiff and hematoxylin-eosin staining of mouse conjunctiva (CNJ) epithelium of the control (left) or scopolamine-treated DE mice (right), magification, 630×. Black arrows indicate the goblet cells; Red arrows indicate the infultrated inflammatory cells. (D) Comparison of the numbers of goblet cells in the superior and inferior bulbar and tarsal CNJ epithelium of BALB/c mice treated with or without scopolamine injection for 5 days. (E) Hematoxylin-eosin staining of mouse CNJ of the control (left) or DE mice (right), magnification, 400×. Red arrows indicate typical infiltrated inflammatory cells. (F) Comparison of the numbers of infiltrated cells: MØ, macrophage; Mono, monocyte; Lymph, lymphocyte. The number of mice used for counting is shown in parentheses. Results are presented as mean ± SD. Statistical significance with Student's t-test analyses is indicated.
Since the loss of CNJ goblet cells is a widely acknowledged character of DE disease in both humans and mice,35,37,43 the goblet cell densities were examined at the superior and inferior bulbar and tarsal CNJ areas. Examination was performed on paraffin-embedded mice eye slides stained with PAS-H&E. Compared to the control mice, the DE mice had significantly reduced the numbers of goblet cells, as indicated by the dark triangles (Fig. 2C) and the bar graph (Fig. 2D). An increase in inflammatory cell infiltration was also observed, as indicated by the red arrows (Fig. 2C) and further confirmed in Figures 2E and 2F. These findings are consistent with those of other researches,31,44 implying the presence of an inflammatory reaction.
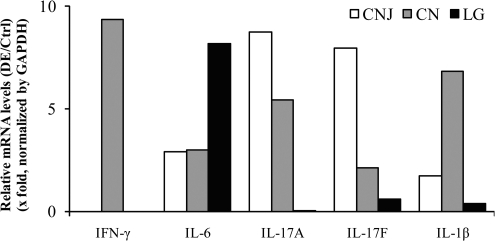
To evaluate the level of ocular surface inflammation in the DE mice, the concentration of inflammatory cytokines in DE mouse tear thread samples was analyzed and compared to that of the control mice (10-Plex Luminex kit). As shown in Table 1, all the tested inflammatory cytokine levels were found significantly elevated in DE mice after 5 days of desiccating treatment, when compared to that of the untreated controls, which showed similar results between day 1 and day 6. Results from Table 1 also imply a mixed immune response to the desiccation stress, involving elevations in Th1-, Th2-, and Th17-related cytokines, with greater elevations in Th1-related (such as IFN-γ, TNF-α, IL-12, average elevation: 4.89 ± 0.38-fold) and Th17-related (such as IL-6 and IL-17; average elevation, 5.14 ± 0.21-fold) cytokines, and lower elevations in Th2-related (such as IL-4, IL-5, and IL-13; average elevation, 4.72 ± 0.10-fold) cytokines. The differences of Th2 to Th1 or Th2 to Th17 cytokine upregulations in our BALB/c DE mice appear to be smaller than those previous published data using C57BL/6 mice; they may reflect the finding that BALB/c mice are more prone to Th2-like immune responses.32,37 Nevertheless, our results are consistent with the trend that DE elicits a mixed T-helper cell-related immune response45,46 and that Th17 cells are the dominant pathogenic effectors in DE disease.27,42,46 Based on these results, a selected panel of cytokines with a fivefold increase in tears, IFN-γ, IL-6, IL-17A (or IL-17), IL-17F, and IL-1β, were assayed for levels of gene transcription as measured by qRT2-PCR. This was confirmed to be the result of increased cytokine gene transcription using RNA samples from the CNJ, CN, and lacrimal glands (LG) of DE mice, compared with control mice (Fig. 3), suggesting that the ocular surface of the DE mice was inflamed.
Table 1.
The Effect of Desiccation on Tear Inflammatory Cytokine Production
| Gene | Control (pg/mm) |
DE (pg/mm) |
Relative Fold Increase (DE/Ctrl)† | ||
|---|---|---|---|---|---|
| Day 1* | Day 6* | Day 1* | Day 6* | ||
| IL-4 | 2.36 ± 0.19 | 1.80 ± 0.16 | 2.00 ± 0.24 | 7.04 ± 0.61 | 4.60** |
| IL-5 | 1.32 ± 0.11 | 1.06 ± 0.08 | 1.15 ± 0.12 | 4.42 ± 0.28 | 4.79*** |
| IL-6 | 7.95 ± 0.56 | 5.81 ± 0.43 | 6.90 ± 0.71 | 26.67 ± 1.86 | 5.29*** |
| IL-12 (p70) | 7.32 ± 0.45 | 5.76 ± 0.44 | 6.43 ± 0.64 | 22.50 ± 1.37 | 4.45*** |
| IL-13 | 78.63 ± 6.18 | 57.42 ± 6.37 | 70.43 ± 8.02 | 245.48 ± 21.09 | 4.77*** |
| IL-17 | 2.56 ± 0.18 | 1.91 ± 0.17 | 2.21 ± 0.26 | 8.24 ± 0.38 | 4.99*** |
| IFN-γ | 19.71 ± 1.51 | 14.90 ± 1.20 | 17.04 ± 1.95 | 65.35 ± 4.10 | 5.07*** |
| TNF-α | 8.46 ± 0.92 | 4.96 ± 0.98 | 6.84 ± 1.37 | 20.66 ± 2.18 | 5.15** |
| IL-1β | 21.18 ± 1.94 | 17.55 ± 1.97 | 17.78 ± 1.71 | 68.40 ± 7.46 | 4.64*** |
Tear cytokines of scopolamine-treated groups (dry eye [DE]) and control groups (Ctrl) were measured by Luminex kit. A representative of four independent experiments is shown.
Data are shown as mean ± SE of the cytokine concentration (picogram) per millimeter of the wetted length of tear threads for each mouse, n = 6 for DE and n = 9 for controls.
Two-tailed unequal-variance Student's t-tests were used to compare the ratio of each cytokine measurement between treated mice at day 6 vs. day 1 and those of the untreated mice,
P < 0.05,
P < 0.01.
Figure 3.
qRT2-PCR assays of selected cytokine genes on RNA samples from scopolamine-treated mice or controls (n = 5 per group). CN, cornea; CNJ, conjunctiva; LG, lacrimal gland.
The Ocular Surface of Treated Mice Had Higher Expression of sPLA2-IIa
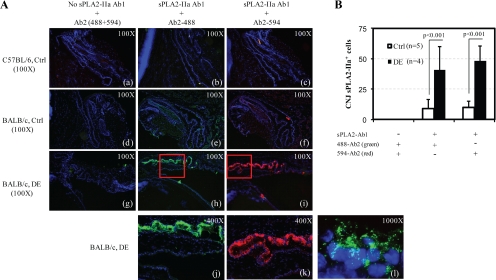
sPLA2-IIa has been shown to be associated with many human inflammatory diseases, including DE disease,22 yet no studies have been attempted to associate sPLA2-IIa with DE disease in mice. Therefore, the changes of sPLA2-IIa expression in the scopolamine-treated mice versus the untreated controls were examined, using immunofluorescence analyses with sPLA2-IIa-specific antibody for primary staining and two-color FITC-labeled antibodies for secondary staining. As shown in Figure 4A, the BALB/c controls without the sPLA2-IIa primary antibody staining or the C57BL/6 controls with or without primary sPLA2-IIa antibody showed negative staining. Under the same condition, the CNJ epithelia layer of the control mice exhibited moderate fluorescent staining (Fig. 4Ae-f), while the CNJ epithelia layer of DE mice exhibited much heavier staining (Fig. 4Ah–k and 4B), both in goblet cells and in epithelial cells. The two secondary antibodies resulted in similar staining patterns. Therefore, we can conclude that the desiccation stress induced higher levels of sPLA2-IIa expression in the DE-CNJ epithelia. Furthermore, the sPLA2-IIa staining appeared as a granular pattern inside cells, more concentrated near the nucleus, and fanning out toward the cell membrane (Fig. 4A-l). In both DE and control mice, neither the CN epithelium nor the LG exhibited any sPLA2-IIa-specific staining related to those of the CNJ.
Figure 4.
Immunofluorescence (IF) assays of CNJ cryosections from scopolamine-treated mice (g–l) and control mice (a–c for C57BL/6 mice control, d–f for BALB/c mice control) stained with polyclonal goat anti-mouse sPLA2-IIa IgG (primary antibody or Ab1), followed by mouse/human-absorbed FITC-labeled polyclonal donkey anti-goat IgG (secondary antibody or Ab2; the Alexa Fluor-488–labeled Ab2 is shown in green, the Alexa Fluor-594–labeled Ab2 shown in red), and counterstained with 4′-6-Diamidino-2-phenylindole (DAPI, shown in blue) to visualize nuclei. The j–k are the higher-magnification images of the insets in h–i. Magnification: a–i, 100×; j–k, 400×; l, 1000×.
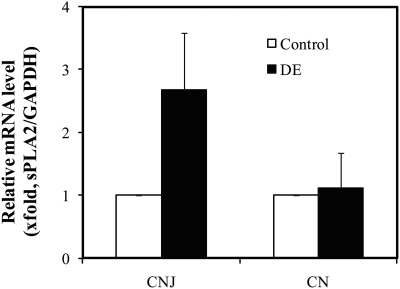
The messenger RNA of sPLA2-IIa was examined by qRT2-PCR. As shown in Figure 5, the expression of pla2g2a mRNA was increased nearly threefold in CNJ of DE mice compared to the controls. In both DE and control mice, no changes were observed in the CN epithelia, supporting our immunofluorescence observations. This line of evidence strongly implies that the increased levels of sPLA2-IIa in CNJ during ocular surface inflammation in DE disease is due to an increased expression of the pla2g2a gene, and not simply a sPLA2-IIa concentration increase resulting from tear deficiency. This is consistent with our results from human subjects with DE disease (unpublished results).
Figure 5.
RT2-PCR of sPLA2-IIa mRNA expression on the ocular surface. CNJ or CN samples from control or DE mice were pooled per group (n = 5) for total RNA isolation and purification. A total of four groups of mice from control or DE mice were assayed. Ten (10) ng of total RNA isolated from CNJ, CN, or LG were reverse transcribed and used for RT2-PCR. Each pooled total RNA sample ran 6 repeats of RT2-PCR with sPLA2-IIa primers, and results were normalized with GAPDH. The results are presented as a mean value ± SD.
sPLA2-IIa Amplified Production of Inflammatory Mediator PGE2 in Sensitized CNJ Organ Cultures
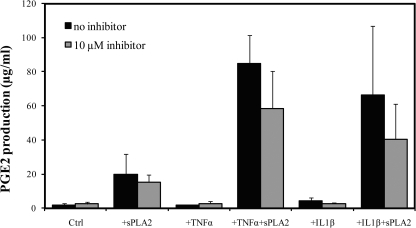
We next tested whether or not the elevated expression of sPLA2-IIa in the CNJ of DE mice could amplify ocular surface inflammation. We evaluated this amplification by measuring production of the inflammatory mediator PGE2 released in the media. We hypothesized that tear sPLA2-IIa amplifies the inflammatory response when the ocular surface tissue is sensitized. First, we reexamined CNJ from untreated BALB/c mice for their responses to the addition of human recombinant sPLA2-IIa. Consistent with our previous findings,22 the addition of exogenous human recombinant sPLA2-IIa stimulated a relatively low level of PGE2 production (Fig. 6, black bars of the first and second panel).
Figure 6.
sPLA2-IIa amplified PGE2 productions. Conjunctiva from untreated normal mice were preincubated in 200 μL Shem's medium with 5% FBS at 37°C and 95% air and 5% CO2 for 1 hour. After the preincubation, medium was changed to either 10 ng/mL TNF-α or 10 ng/mL IL-1β at designated wells for a 1-hour presensitization. Then the CNJ organs were treated for additional 12 hours with fresh Shem's medium containing either human recombinant sPLA2-IIa, TNF-α, and IL-1β alone, or the combinations of sPLA2-IIa with TNF-α or sPLA2-IIa with IL-1β, plus indicated amount of S3319 inhibitor. Each treatment group contained at least two repeats. Each experiment was measured at least three times for PGE2 production. Results of one representative experiment were plotted. All values are the mean ± SD.
To confirm that the low level of PGE2 production was mainly stimulated by sPLA2-IIa, a highly specific sPLA2-IIa inhibitor, S-3319, was presented to the CNJ together with sPLA2-IIa. S-3319 is reported to block the enzymatic activity of sPLA2-IIa via highly selective binding to the active center of sPLA2-IIa, with an IC50 as low as 29 nM in vivo in rats.14,47,48 However, few studies were done on mice CNJ. As shown in Figure 6 (panel 2, gray bars), the addition of 10 μM S-3319 with 25 μg/mL sPLA2-IIa showed a nearly 30% decrease in sPLA2-IIa–induced PGE2 production.
Previously we have shown that the sPLA2-IIa stimulated PGE2 production of normal CNJ organs of BALB/c mice in a TNF-α–dependent manner.22 Therefore, in this study we investigated whether presensitizing normal untreated BALB/c CNJ organs with another proinflammatory cytokine IL-1β would likewise cause a drastic PGE2 release in response to exogenous sPLA2-IIa. As shown in Figure 6 (panel 3 and 5, black bars), the addition of IL-1β or TNF-α alone caused minimal to low levels of PGE2 production, and the addition of S-3319 inhibitor showed no inhibitory effects compared with those of the controls. However, when sPLA2-IIa was added to the CNJ pretreated with IL-1β or TNF-α (panel 4 and 6, black bars), PGE2 production was greatly increased (85-fold for sPLA2+TNF-α, and 66-fold for sPLA2+IL-1β). These findings suggest that the presensitization of the ocular surface is necessary for sPLA2-IIa to trigger a significant amplification of ocular surface inflammation. Again, the addition of 10 μM S3319 effectively reversed the PGE2 amplification triggered by the exogenous sPLA2-IIa (31% reduced in for sPLA2+TNF-α group, and 39% reduced for sPLA2+IL-1β).
Since the desiccation induced by scopolamine-air ventilation caused an elevation of ocular surface inflammation and sPLA2-IIa expression (as shown in our BALB/c mice model in Figures 2–5 and Table 1), we tested whether or not the imposed desiccation stress on CNJ can act as another presensitizing agent to amplify PGE2 production when exogenous sPLA2-IIa was added (as shown in TNF-α or IL-1β presensitization). We further tested whether or not this PGE2 amplification is reversible by S-3319 inhibition. As shown in Table 2, PGE2 production showed no significant changes on the addition of increasing amount of inhibitor alone, either normal CNJ or DE-CNJ. On addition of 25 μg/mL sPLA2-IIa, PGE2 production was stimulated over fourfold to the CNJ from normal mice and sevenfold to the CNJ from DE mice. PGE2 release was nearly 2 times more up than that of undesiccated controls. In all groups of CNJ, either from normal untreated mice or DE mice, PGE2 was significantly increased in response to the exogenous sPLA2-IIa treatment (P < 0.005). Moreover, compared to Ctrl-CNJ, the treatment of DE-CNJ with S-3319 showed clearly significant inhibitory effect on PGE2 production in an apparently dose-dependent manner. For example, a lower dose of S-3319 inhibitor treatment (0.08 μM) reduced PGE2 production by 40%. When the higher dose of S-3319 (10 μM) was present, sPLA2-IIa-stimulated PGE2 amplification was significantly reduced by 69%. These results suggest that the compromised CNJ induced by desiccation stress was more sensitive than Ctrl-CNJ to the changes of the enzymatically active form of sPLA2-IIa in mediating desiccation-induced PGE2 production and inflammation.
Table 2.
The Effect of sPLA2-IIa Inhibitor on PGE2 Production of BALB/c Mice Conjunctiva Cultures
| Inhibitor (μM) | −sPLA2-IIa |
+sPLA2-IIa |
Ratio of ±sPLA2-IIa (×fold)† | P (<) (± Inhibitor)‡ | P (<) (± sPLA2-IIa)§ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (μg/mL)* | SD | Mean (μg/mL)* | SD | |||||||
| Ctrl-CNJ | 0.00 | 4.33 | ± | 0.10 | 18.10 | ± | 0.91 | 4.18 | 0.005 | |
| 0.08 | 4.68 | ± | 0.22 | 11.13 | ± | 0.71 | 2.38 | 0.005 | 0.005 | |
| 0.40 | 4.39 | ± | 0.17 | 12.59 | ± | 1.67 | 2.87 | 0.005 | 0.005 | |
| 2.00 | 5.61 | ± | 0.19 | 12.59 | ± | 1.11 | 2.25 | 0.005 | 0.005 | |
| 10.00 | 4.27 | ± | 0.20 | 14.52 | ± | 0.58 | 3.40 | 0.005 | 0.005 | |
| DE-CNJ | 0.00 | 3.54 | ± | 0.18 | 24.26 | ± | 2.16 | 6.85 | 0.005 | |
| 0.08 | 3.79 | ± | 0.18 | 14.66 | ± | 0.78 | 3.86 | 0.005 | 0.005 | |
| 0.40 | 6.50 | ± | 1.76 | 16.13 | ± | 1.59 | 2.48 | 0.005 | 0.005 | |
| 2.00 | 3.58 | ± | 0.17 | 21.15 | ± | 0.23 | 5.90 | 0.025 | 0.005 | |
| 10.00 | 4.27 | ± | 0.20 | 7.88 | ± | 0.17 | 1.85 | 0.005 | 0.005 | |
All values are mean ± SD (μg/mL) of two measurements, n = 4 eyes in each group.
Ratio was calculated using PGE2 produced by conjunctiva (CNJ) from normal or dry eye (DE) mice treated with sPLA2-IIa (25 μg/mL) against samples at the same indicated amount of inhibitor. Ctrl-CNJ, conjunctiva from untreated mice; DE-CNJ, conjunctiva from mice treated with scopolamine-air ventilation.
Student's t-test was run to evaluate the effect of the inhibitor on PGE2 production at the groups with an indicated inhibitor concentration to the groups without inhibitor.
Student's t-test was run to evaluate the effect of sPLA2 on PGE2 production between groups with added sPLA2-IIa and groups without sPLA2-IIa at each inhibitor level.
Discussion
In our present study, we have demonstrated that the scopolamine-air ventilation treatments are successful in inducing DE-like syndrome in BALB/c mice: (1) decreased tear production, (2) increased fluorescein staining indicating “damage” on the CN epithelia, (3) increased inflammatory cell filtration in the CNJ epithelia, (4) decreased goblet cell density in the CNJ epithelia, (5) increased inflammatory cytokines in tears, and (6) increased cytokine mRNA expression in the CNJ epithelia.
Accumulated evidence from both human and animal DE subjects implies that DE disease is likely a T-helper cell (Th)–mediated autoimmune-related inflammatory disorder. For example, an increased expression of Th17 inducers, such as IL-23, IL-17, and IFN-γ, has been found in CNJ of DE patients. And IL-17-specific antibody neutralization can ameliorate DE-induced CN barrier dysfunction.45 Therefore, Th17 is suggested to be the dominant pathogenic effector in DE pathogenesis.27 Consistent with this assumption, our results showed that not only were Th17-associated cytokine concentrations the most elevated (compared to Th1 and Th2 cytokines) in the tears of DE mice (Table 1), but also that their gene expression was the most increased in the CN and CNJ. We also detected an increased IFN-γ gene expression in CN and IL-6 gene expression in LG of the DE mice. IFN-γ is produced by Th1 cells, IL-6 is produced by several cell types including antigen presenting cells and Th2 cells, and IL-17A and IL-17F are produced by Th17 cells. These cytokines either serve as inducers to Th17 T-cell differentiation (IFN- γ, IL-6, and IL-1 β) or are co-produced by Th17 T-cells themselves (IL-17A and IL-17F).45 IL-17F was chosen as part of the cytokine panel because it is the closest homolog to IL-17A among the Th17-associated cytokines and is shown to play a critical role in regulating the inflammatory response.49 Although IL-17A and IL-17F share many similarities—both are encoded at the same chromosome locus, coexpressed in Th-17 cells, and may use the same signaling pathways to induce similar downstream inflammatory genes—they function differently in the setting of inflammation.49 For example, although desiccation significantly induces both IL-17A and IL-17F expression in CN and CNJ of C57BL/6 DE mice, the induced levels are quite different, especially in IL-2rα-/- mice (CD25OK).45,46 Furthermore, in normal media containing Th17-inducing cytokines, the CD4+ T-cells isolated from C57BL/6 express higher levels of IL-17F than IL-17A in vitro; whereas in a 50% conditioned media from human CN culture, the same CD4+ T-cells express much higher levels of IL-17A than IL-17F on stimulation.50 IL-17F gene polymorphisms are also reported to account for the eye lesions of patients with Behçet's disease, a chronic multisystem inflammatory disorder characterized by recurrent oral aphthous ulcers, genital ulcers, uveitis, and skin lessons.51
Since the concentration and activity of sPLA2-IIa in human tears have been reported as very high, we wanted to use a model that would allow us to further evaluate the role of sPLA2 in ocular surface inflammation. The DE model was therefore chosen as a well-recognized external inflammatory model associated with ocular surface disease. Although both BALB/c mice and C57BL/6 mice are reported to be useful in developing DE disease models, as well as other disease models,17,52,53 only BALB/c mice have a functional pla2g2g gene. The fact that C57BL/6 mice do not have a functional pla2g2g gene suggests that other isoforms of the sPLA2 family are capable of compensating for sPLA2-IIa in the development of DE disease.54 Studies of the distribution patterns and roles of the different sPLA2 isoforms in DE pathogenesis are under way.
Our immunofluorescence staining results revealed that the sPLA2-IIa expression increased exclusively in DE-CNJ, in the goblet as well as the epithelial cells. This increase in sPLA2-IIa appears to be associated with signs and symptoms of DE disease and other inflammatory biomarkers, such as increased inflammatory cell infiltration, elevated tear cytokines, and elevated CNJ cytokine mRNA. sPLA2-IIa mRNA analyses support that this is an active process of sPLA2-IIa secretion in DE-CNJ, not simply an evaporation effect. The sPLA2-IIa expression was not detected for any change in CN, neither by IF nor by (q)RT2-PCR analyses, consistent with our IF staining results and Turner's findings using human CN epithelia (2007).21 The sPLA2-IIa-specific signals were also not detected any changes in the LG samples. These findings suggest that sPLA2-IIa comes primarily from CNJ, not from CN or LG. We will confirm these findings using more sensitive methods in the near future.
Our previous work with mouse CNJ culture demonstrated an amplified inflammatory response with addition of sPLA2-IIa to the BALB/c mouse CNJ tissues presensitized with TNF-α.22 sPLA2-IIa alone stimulated a low level of PGE2 production, whereas TNF-α alone showed an even smaller effect than sPLA2-IIa alone; however, sPLA2-IIa added to CNJ that was presensitized by TNF-α showed a near 10-fold increase in PGE2 production. After this, two other common presensitizing reagents, IL-1β and desiccation, were tested side by side with TNF-α. The desiccated CNJ was obtained from our DE model mice. Both IL-1β and desiccation alone induced minimal to lower levels of PGE2 production, similar to that observed from sPLA2-IIa or TNF-α alone. However, in combination with sPLA2-IIa, significant amplification of PGE2 production was observed in both CNJ pretreated with IL-1β and desiccated CNJ. Furthermore, PGE2 levels, amplified by the different combinations of proinflammatory cytokines with sPLA2-IIa or desiccation stress with sPLA2-IIa, were significantly reduced by sPLA2-IIa-specific inhibition, indicating that sPLA2-IIa plays an important role in amplifying ocular surface inflammation.
Our collective mouse and human DE disease results from this study and our previous reports22,40 suggest that sPLA2-IIa plays an important role in amplifying the inflammatory response on the ocular surface, specifically in DE disease and likely in other inflammatory diseases as well. Furthermore, the results show that there is an increase in both concentration and activity of sPLA2-IIa in association with typical signs and symptoms of DE disease. The changes in sPLA2-IIa correlate with increases of other inflammatory biomarkers seen in human and mouse DE disease.40 With the present study we set out to show that tear sPLA2-IIa leads to an amplification of the inflammatory response on the ocular surface when the surface cells are compromised, such as in DE disease and other inflammatory diseases.
In summary, we demonstrate that BALB/c mice treated with scopolamine-air ventilation can develop a DE disease model with tear deficiency, ocular surface inflammation, and sPLA2-IIa elevation. We also demonstrate a role of sPLA2-IIa in amplifying ocular surface inflammation, especially when the ocular surface is presensitized or compromised. This amplification can be reversed by sPLA2-IIa-specific inhibition. The inhibitors provide a useful tool to evaluate the role of sPLA2 in inflammation of the ocular surface. Furthermore, sPLA2 inhibition may be useful in controlling or preventing inflammation of ocular surface in external diseases. Future work will evaluate sPLA2 and the inhibitors in vivo and using knockout mice.
Acknowledgments
The authors thank Alexander Pinhas for his assistance in manuscript preparation; Libang Zhang, Emma Farfan, and Ying Dai from the Animal Facility of Mount Sinai Medical Center for running the Luminex 100 system; and Qiang Zhang from Schepens Eye Research Institute, Harvard Medical School, for providing useful information for the specially designed mouse cage construction.
Footnotes
Supported in part by the Martin and Toni Sosnoff Foundation and by NIH/NCCAM center Grant no. P01 AT002647-01 (X-ML).
Disclosure: Y. Wei, None; S.P. Epstein, None; S. Fukuoka, None; N.P. Birmingham, None; X.-M. Li, None; P.A. Asbell, None
References
- 1. Rosenson RS, Gelb MH. Secretory phospholipase A2: a multifaceted family of proatherogenic enzymes. Curr Cardiol Rep. 2009;11:445–451 [DOI] [PubMed] [Google Scholar]
- 2. Tischfield JA, Xia YR, Shih DM, et al. Low-molecular-weight, calcium-dependent phospholipase A2 genes are linked and map to homologous chromosome regions in mouse and human. Genomics. 1996;32:328–333 [DOI] [PubMed] [Google Scholar]
- 3. Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–1259 [DOI] [PubMed] [Google Scholar]
- 4. Jaworski K, Ahmadian M, Duncan RE, et al. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat Med. 2009;15:159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50(Suppl):S237–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bidgood MJ, Jamal OS, Cunningham AM, Brooks PM, Scott KF. Type IIA secretory phospholipase A2 up-regulates cyclooxygenase-2 and amplifies cytokine-mediated prostaglandin production in human rheumatoid synoviocytes. J Immunol. 2000;165:2790–2797 [DOI] [PubMed] [Google Scholar]
- 7. Karakas M, Koenig W. Varespladib methyl, an oral phospholipase A2 inhibitor for the potential treatment of coronary artery disease. IDrugs. 2009;12:585–592 [PubMed] [Google Scholar]
- 8. Masuda S, Murakami M, Komiyama K, et al. Various secretory phospholipase A2 enzymes are expressed in rheumatoid arthritis and augment prostaglandin production in cultured synovial cells. FEBS Lett J. 2005;272:655–672 [DOI] [PubMed] [Google Scholar]
- 9. Hurt-Camejo E, Camejo G, Peilot H, Oorni K, Kovanen P. Phospholipase A(2) in vascular disease. Circ Res. 2001;89:298–304 [DOI] [PubMed] [Google Scholar]
- 10. Minami T, Tojo H, Shinomura Y, Matsuzawa Y, Okamoto M. Increased group II phospholipase A2 in colonic mucosa of patients with Crohn's disease and ulcerative colitis. Gut. 1994;35:1593–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cai TQ, Thieblemont N, Wong B, Thieringer R, Kennedy BP, Wright SD. Enhancement of leukocyte response to lipopolysaccharide by secretory group IIA phospholipase A2. J Leukoc Biol. 1999;65:750–756 [DOI] [PubMed] [Google Scholar]
- 12. Bowton DL, Seeds MC, Fasano MB, Goldsmith B, Bass DA. Phospholipase A2 and arachidonate increase in bronchoalveolar lavage fluid after inhaled antigen challenge in asthmatics. Am J Respir Crit Care Med. 1997;155:421–425 [DOI] [PubMed] [Google Scholar]
- 13. Boilard E, Lai Y, Larabee K, et al. A novel anti-inflammatory role for secretory phospholipase A2 in immune complex-mediated arthritis. EMBO Mol Med. 2010;2:172–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Titsworth WL, Cheng X, Ke Y, et al. Differential expression of sPLA2 following spinal cord injury and a functional role for sPLA2-IIA in mediating oligodendrocyte death. Glia. 2009;57:1521–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Birts CN, Barton CH, Wilton DC. Catalytic and non-catalytic functions of human IIA phospholipase A2. Trends Biochem Sci. 2010;35:28–35 [DOI] [PubMed] [Google Scholar]
- 16. Mirtti T, Laine VJ, Hiekkanen H, et al. Group IIA phospholipase A as a prognostic marker in prostate cancer: relevance to clinicopathological variables and disease-specific mortality. APMIS. 2009;117:151–161 [DOI] [PubMed] [Google Scholar]
- 17. Fijneman RJ, Bade LK, Peham JR, et al. Pla2g2a attenuates colon tumorigenesis in azoxymethane-treated C57BL/6 mice; expression studies reveal Pla2g2a target genes and pathways. Cell Oncol. 2009;31:345–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mannello F, Qin W, Zhu W, Fabbri L, Tonti GA, Sauter ER. Nipple aspirate fluids from women with breast cancer contain increased levels of group IIa secretory phospholipase A2. Breast Cancer Res Treat. 2008;111:209–218 [DOI] [PubMed] [Google Scholar]
- 19. Aho VV, Nevalainen TJ, Saari KM. Group IIA phospholipase A2 content of tears in patients with keratoconjunctivitis sicca. Graefes Arch Clin Exp Ophthalmol. 2002;240:521–523 [DOI] [PubMed] [Google Scholar]
- 20. Qu XD, Lehrer RI. Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram-positive bacteria in human tears. Infect Immun. 1998;66:2791–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turner HC, Budak MT, Akinci MA, Wolosin JM. Comparative analysis of human conjunctival and corneal epithelial gene expression with oligonucleotide microarrays. Invest Ophthalmol Vis Sci. 2007;48:2050–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen D, Wei Y, Li X, Epstein S, Wolosin JM, Asbell P. sPLA2-IIa is an inflammatory mediator when the ocular surface is compromised. Exp Eye Res. 2009;88:880–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DEWS The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop. (2007). Ocul Surf. 2007;5:75–92 [DOI] [PubMed] [Google Scholar]
- 24. Hori Y, Spurr-Michaud SJ, Russo CL, Argueso P, Gipson IK. Effect of retinoic acid on gene expression in human conjunctival epithelium: secretory phospholipase A2 mediates retinoic acid induction of MUC16. Invest Ophthalmol Vis Sci. 2005;46:4050–4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Niederkorn JY. Emerging concepts in CD8(+) T regulatory cells. Curr Opin Immunol. 2008;20:327–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abelson MB, Ousler GW, 3rd, Maffei C. Dry eye in 2008. Curr Opin Ophthalmol. 2009;20:282–286 [DOI] [PubMed] [Google Scholar]
- 27. Chauhan SK, Dana R. Role of Th17 cells in the immunopathogenesis of dry eye disease. Mucosal Immunol. 2009;2:375–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pflugfelder SC, de Paiva CS, Li DQ, Stern ME. Epithelial-immune cell interaction in dry eye. Cornea. 2008;27(Suppl 1):S9–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pflugfelder SC, Stern ME. Immunoregulation on the ocular surface: 2nd Cullen Symposium. Ocul Surf. 2009;7:67–77 [DOI] [PubMed] [Google Scholar]
- 30. Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301 [DOI] [PubMed] [Google Scholar]
- 31. Barabino S, Shen L, Chen L, Rashid S, Rolando M, Dana MR. The controlled-environment chamber: a new mouse model of dry eye. Invest Ophthalmol Vis Sci. 2005;46:2766–2771 [DOI] [PubMed] [Google Scholar]
- 32. Corrales RM, Villarreal A, Farley W, Stern ME, Li DQ, Pflugfelder SC. Strain-related cytokine profiles on the murine ocular surface in response to desiccating stress. Cornea. 2007;26:579–584 [DOI] [PubMed] [Google Scholar]
- 33. Yeh S, de Paiva CS, Hwang CS, et al. Spontaneous T cell mediated keratoconjunctivitis in Aire-deficient mice. Br J Ophthalmol. 2009;93:1260–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yoon KC, De Paiva CS, Qi H, et al. Desiccating environmental stress exacerbates autoimmune lacrimal keratoconjunctivitis in non-obese diabetic mice. J Autoimmun. 2008;30:212–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Paiva CS, Villarreal AL, Corrales RM, et al. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest Ophthalmol Vis Sci. 2007;48:2553–2560 [DOI] [PubMed] [Google Scholar]
- 36. Strong B, Farley W, Stern ME, Pflugfelder SC. Topical cyclosporine inhibits conjunctival epithelial apoptosis in experimental murine keratoconjunctivitis sicca. Cornea. 2005;24:80–85 [DOI] [PubMed] [Google Scholar]
- 37. Barabino S, Rolando M, Chen L, Dana MR. Exposure to a dry environment induces strain-specific responses in mice. Exp Eye Res. 2007;84:973–977 [DOI] [PubMed] [Google Scholar]
- 38. Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995;21:221–232 [PubMed] [Google Scholar]
- 39. Scifo C, Barabino S, De Pasquale G, Blanco AR, Mazzone MG, Rolando M. Effects of a new lipid tear substitute in a mouse model of dry eye. Cornea. 2010;29:802–806 [DOI] [PubMed] [Google Scholar]
- 40. Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28:1023–1027 [DOI] [PubMed] [Google Scholar]
- 41. Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjögren's Syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006;176:3950–3957 [DOI] [PubMed] [Google Scholar]
- 42. Chauhan SK, El Annan J, Ecoiffier T, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182:1247–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lopin E, Deveney T, Asbell PA. Impression cytology: recent advances and applications in dry eye disease. Ocul Surf. 2009;7:93–110 [DOI] [PubMed] [Google Scholar]
- 44. Chen W, Zhang X, Zhang J, et al. A murine model of dry eye induced by an intelligently controlled environmental system. Invest Ophthalmol Vis Sci. 2008;49:1386–1391 [DOI] [PubMed] [Google Scholar]
- 45. De Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2:243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. De Paiva CS, Hwang CS, Pitcher JD, 3rd, et al. Age-related T-cell cytokine profile parallels corneal disease severity in Sjögren's syndrome-like keratoconjunctivitis sicca in CD25KO mice. Rheumatology (Oxford) 2010;49:246–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arumugam TV, Arnold N, Proctor LM, et al. Comparative protection against rat intestinal reperfusion injury by a new inhibitor of sPLA2, COX-1 and COX-2 selective inhibitors, and an LTC4 receptor antagonist. Br J Pharmacol. 2003;140:71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hansford KA, Reid RC, Clark CI, et al. D-Tyrosine as a chiral precusor to potent inhibitors of human nonpancreatic secretory phospholipase A2 (IIa) with antiinflammatory activity. Chembiochem. 2003;4:181–185 [DOI] [PubMed] [Google Scholar]
- 49. Yang XO, Chang SH, Park H, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zheng X, Bian F, Ma P, et al. Induction of Th17 differentiation by corneal epithelial-derived cytokines. J Cell Physiol. 2010;222:95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jang WC, Nam YH, Ahn YC, et al. Interleukin-17F gene polymorphisms in Korean patients with Behcet's disease. Rheumatol Int. 2008;29:173–178 [DOI] [PubMed] [Google Scholar]
- 52. Novelli MR, Wasan H, Rosewell I, et al. Tumor burden and clonality in multiple intestinal neoplasia mouse/normal mouse aggregation chimeras. Proc Natl Acad Sci USA. 1999;96:12553–12558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fijneman RJ, Peham JR, van de Wiel MA, et al. Expression of Pla2g2a prevents carcinogenesis in Muc2-deficient mice. Cancer Sci. 2008;99:2113–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sawada H, Murakami M, Enomoto A, Shimbara S, Kudo I. Regulation of type V phospholipase A2 expression and function by proinflammatory stimuli. Eur J Biochem. 1999;263:826–835 [DOI] [PubMed] [Google Scholar]