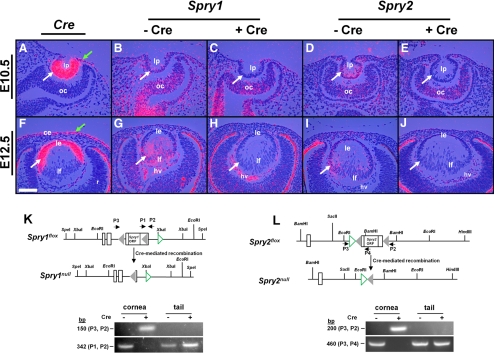
Figure 3.
Conditional deletion of Spry1 and -2 in the lens. In situ hybridization (A–J) was performed with 35S-labeled Cre and Spry1 and -2 riboprobes on sections of Cre transgenic (A, F) and Spry1 (B–H) and Spry2 (D–J) mutant embryos. Cre recombinase was expressed in the lens pit (A, white arrow), lens epithelium (F), and presumptive corneal epithelial cells (A, F, green arrows) in the Le-Cre mice, as reported previously. Cre+ embryos showed loss of Spry1 (C, H) and Spry2 (E, J) expression in the lens but not in the optic cup (oc) (B–E) or the hyaloid vasculature (hv) (G–J) where Cre recombinase was not expressed. (K, L) Spry1 and -2 floxed alleles (adapted and modified with permission from Basson MA, Akbulut S, Watson-Johnson J, et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell 2005;8:229–239. © Elsevier, and Shim K, Minowada G, Coling DE, Martin GR. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev Cell 2005;8:553–564. © Elsevier). Cre-mediated recombination in the cornea was determined by PCR using primers (P1, P2, and P3) flanking the loxP sequences (gray triangles) of Spry1 and -2 genes. Tail genomic DNA was used as negative controls. Open rectangles: exons. Green open triangles: frt sequences. After recombination, P2 and P3 amplicons were seen in Cre+ corneas but not in Cre− corneas, as they are too large to be amplified. ce, corneal epithelium; le, lens epithelium; lf, lens fibers; lp, lens pit; ORF, open reading frame; r, retina. Scale bar, 20 μm.