Abstract
Thymineless death (TLD) is the rapid loss of viability in bacterial, yeast, and human cells starved of thymine. TLD is the mode of action of common anticancer drugs and some antibiotics. TLD in Escherichia coli is accompanied by blocked replication and chromosomal DNA loss and recent work identified activities of recombination protein RecA and the SOS DNA-damage response as causes of TLD. Here, we examine the basis of hypersensitivity to thymine deprivation (hyper-TLD) in mutants that lack the UvrD helicase, which opposes RecA action and participates in some DNA repair mechanisms, RecBCD exonuclease, which degrades double-stranded linear DNA and works with RecA in double-strand-break repair and SOS induction, and RuvABC Holliday-junction resolvase. We report that hyper-TLD in ∆uvrD cells is partly RecA dependent and cannot be attributed to accumulation of intermediates in mismatch repair or nucleotide-excision repair. These data imply that both its known role in opposing RecA and an additional as-yet-unknown function of UvrD promote TLD resistance. The hyper-TLD of ∆ruvABC cells requires RecA but not RecQ or RecJ. The hyper-TLD of recB cells requires neither RecA nor RecQ, implying that neither recombination nor SOS induction causes hyper-TLD in recB cells, and RecQ is not the sole source of double-strand ends (DSEs) during TLD, as previously proposed; models are suggested. These results define pathways by which cells resist TLD and suggest strategies for combating TLD resistance during chemotherapies.
BACTERIAL, yeast, and human cells deprived of thymine rapidly lose the ability to form colonies, a phenomenon known as thymineless death (TLD) (Barner and Cohen 1954; Ahmad et al. 1998). TLD is the mode of action of several common chemotherapeutic drugs including anticancer agents 5-fluorouracil (5-FU), raltitrexed (Tomudex), (Takemura and Jackman 1997) and methotrexate, and the antibiotic trimethoprim (Mcguire 2003). Yet until recently and despite extensive study, how TLD occurs remained elusive. TLD occurs in replicating cells (e.g., Cummings and Kusy 1970), probably because of DNA damage sustained during replication in the absence of thymine. First, sedimentation analysis revealed accumulation of single-strand (ss)-DNA breaks in plasmid (Freifelder 1969) and chromosomal (Nakayama and Hanawalt 1975) DNA following thymine deprivation. Second, pulsed-field-gel electrophoresis and electron microscopy showed aberrant DNA structures containing large (∼1–3 kb) regions of ssDNA (Nakayama et al. 1994). Implicating replication-generated DNA damage, recent work from the Khodursky group and our laboratory using DNA microarrays and fluorescent in situ hybridization (FISH) revealed that Escherichia coli cells undergoing TLD specifically lose origin-proximal DNA sequences early during thymine deprivation (Fonville et al. 2010a; Sangurdekar et al. 2010), followed by loss of replication-terminus-proximal DNA after extended thymine deprivation (Fonville et al. 2010a). Such DNA loss would be expected to contribute to death.
An important window on TLD mechanisms has been afforded by analyses of proteins and pathways that promote TLD in E. coli. Homologous recombination (HR) proteins RecF, RecQ, and RecJ are required for TLD (H. Nakayama et al. 1982, 1984; K. Nakayama et al. 1988). Although this would suggest that HR is a major contributor to the lethality observed under thymine deprivation, confusion surrounded the role for the major recombinase, RecA. Although one group reported that RecA promoted TLD (Inouye 1971), others found no role for RecA (Anderson and Barbour 1973; Nakayama et al. 1982). Recent work reexamining the role of RecA has established that, as Inouye reported, RecA is required for a major fraction of TLD (Fonville et al. 2010a; Kuong and Kuzminov 2010), compatible with the hypothesis that HR is part of TLD process(es). Both groups found a small, early anti-TLD role of RecA, seen as increased TLD in ∆recA cells early during thymine starvation, followed by a large pro-TLD role, seen as far less TLD in ∆recA cells later during starvation. The later large pro-TLD role is the one discussed in experiments using ∆recA here.
RecA functions not only in HR but also in induction of the bacterial response to DNA damage: the SOS response (Ennis et al. 1985). Whereas Morganroth and Hanawalt (2006) tested and rejected the hypothesis that the SOS response promotes TLD, recent work from three groups has reversed this conclusion. First, Sangurdekar et al. (2010) found that SOS-controlled genes are upregulated upon thymine deprivation. Second, we (Fonville et al. 2010a) and Kuong and Kuzminov (2010) found that the SOS response is required for one of a few operative TLD pathways in that TLD is blocked by mutations that block SOS-response induction. Whereas Kuong and Kuzminov (2010) suggested that SOS might promote TLD via upregulation of RecA, resulting in increased HR (Kuong and Kuzminov 2010), we showed that SOS-induced levels of RecA did not substitute for a functional SOS response in promoting TLD. Thus, SOS-promoted upregulation of another gene(s) promotes TLD, and we found that the SOS function responsible is SulA, an inhibitor of cell division (Fonville et al. 2010a). Ultimately, we showed that the main function of RecA and an important role of RecF in the mechanisms of TLD is induction of the SOS DNA-damage response and SulA, leading to permanently arrested cell division and thus inability to form colonies during thymine starvation (Fonville et al. 2010a).
The SOS-mediated transcriptional upregulation of SulA underlies one pathway of TLD, which results in at least one log of killing under thymine starvation (Fonville et al. 2010a). Alternatively and simultaneously, we found that RecQ and RecJ promote TLD via a separate pathway independent of RecA/SOS/SulA, causing an additional log of killing (Fonville et al. 2010a). Although these two pathways (RecA/SOS/SulA pathway and RecA-independent, RecQ/RecJ pathway) contribute to TLD, removing both of them did not abolish TLD completely (Fonville et al. 2010a). An additional RecA- and RecQ-independent pathway(s) not yet identified also contributed about one more log of TLD. Thus, at least three pathways underlie TLD: a RecA-SOS-SulA-dependent pathway, a RecA-independent/RecQ/RecJ-dependent pathway, and a third pathway requiring neither RecA/SOS/SulA nor RecQ/RecJ.
Although the recent work of multiple groups has begun to illuminate the pathways of and DNA intermediates that accompany TLD, important enigmas remain, particularly concerning DNA-repair proteins that participate in pathways by which cells resist TLD. Mutants lacking these proteins show faster loss of colony-forming ability during thymine starvation, referred to here as hyper-TLD. For example, RecBCD double-strand exonuclease functions with RecA both in repair of DNA double-strand breaks and double-strand ends (DSBs/DSEs) by HR and in induction of the SOS response by DSB-inducing agents (Mcpartland et al. 1980; Clark and Sandler 1994), and yet, although both HR and SOS induction promote TLD (Fonville et al. 2010a; Kuong and Kuzminov 2010), cells lacking RecBCD are TLD hypersensitive (Nakayama et al. 1982), not resistant as recA cells are. RecBCD creates single-strand DNA onto which it then loads RecA at the start of both HR and SOS induction (Arnold and Kowalczykowski 2000). Perhaps more understandably, RuvABC, a Holliday-junction resolvase (Friedberg et al. 2005) that removes the intermolecular recombination intermediates (IRIs) that RecA creates, promotes TLD resistance in that cells that lack RuvABC are TLD hypersensitive (Fonville et al. 2010a). The hyper-TLD in ruv cells is RecA dependent, implying that RecA-generated IRIs, left unresolved in the absence of Ruv, kill cells. Such “death-by-recombination,” in which unresolved IRIs kill cells by preventing chromosome segregation, was also observed in mutants that promote extra accumulation of HR intermediates (Magner et al. 2007; Fonville et al. 2010b). Finally, UvrD, a helicase with multiple functions in vivo, one of which is to remove RecA from ssDNA (Veaute et al. 2005), also promotes TLD resistance in that uvrD null mutants are TLD hypersensitive (Siegal 1973). Understanding how cells become TLD hypersensitive and defining the pathways and mechanisms of action of the proteins that allow cells to resist TLD is likely to be important to maximizing TLD-inducing chemotherapies and combating resistance. In this study we define pathways by which UvrD, RuvABC, and RecBCD allow cells to resist TLD.
Materials and Methods
Strains used in this study are given in Table 1. P1 transductions were as described (Miller 1972). TLD experiments were as described (Fonville et al. 2010a). Cells were grown to stationary phase in M9 minimal medium with 50 μg/ml thymine, 0.1% glucose, and 0.5% casamino acids (thy+ growth medium) then diluted 1:20 into fresh thy+ growth medium, and incubated at 37° for ∼1 hr 10 min (1 hr 40 min for strains containing a ΔrecB allele) to allow them to exit stationary phase and enter early log phase. We found the timing of incubation of cells prior to resuspension in TLD medium to be critical for seeing consistent levels of TLD. One milliliter of cells was washed twice with M9 with 0.1% glucose and 0.5% casamino acids but lacking thymine (TLD medium), then resuspended in 2 ml of TLD medium at ∼5 × 106 cells/ml and incubated at 37° for 5 hr with aliquots taken and dilutions plated at the indicated times. Colony-forming units (CFU) were scored on a Microbiology International ProtoCOL colony counter after 24 hr at 37°. Longer incubations verified that all CFU were apparent at 24 hr.
Table 1 . E.coli K-12 strains and plasmids used in this study.
| Plasmid/strain | Relevant genotype | Source/reference |
|---|---|---|
| pCP20 | FLP recombinase vector | Datsenko and Wanner (2000) |
| AB1157 | F−thi-1 hisG4 Δ(gpt-proA)62 argE3 thr-1 leuB6 araC14 lacY1 galK2 xylA5 mtl-1 rpsL31 tsx-33 glnV44 rfbC1 mgl-51 rpoS396 kdgK51 | CGSC1157a (Bachmann 1972) |
| AB2497 | AB1157 thyA12 deoB6 | CGSG2497a (Howard-Flanders et al. 1966) |
| BW26355 | BW25113 ΔrecA635::FRTKanFRT | CGSC7651a (Datsenko and Wanner 2000) |
| DM49 | lexA3 | CGSC6368a |
| GY8322 | AB1157 sfiA11 Δ(srlR-recA)306::Tn10 [mini-F K5353 recA+] | S. Sommers (Gif sur Yvette); ENZ280 (Dri et al. 1991) carrying the K5353 mini-F plasmid (Dutreix et al. 1989) |
| JW2703 | ΔmutS::FRTKanFRT | Baba et al. (2006) |
| JW2860 | ΔrecJ::FRTKanFRT | Baba et al. (2006) |
| MG1655 | Sequenced wild-type E. coli K-12 F− λ− | Blattner et al. (1997) |
| RTC0013 | MG1655 ΔrecB::Kan | Cirz et al. (2005) |
| SMR85 | recA801 srlC300::Tn10 | Lab Collection |
| SMR6201 | R594 ΔrecQ1801::FRTcatFRT | Lopez et al. (2005) |
| SMR8097 | FC40 ΔrecF1804::FRTKanFRT | Pennington and Rosenberg (2007) |
| SMR8547 | MG1655 ΔuvrA402::Gm | Lab collection |
| SMR8548 | MG1655 ΔuvrC403::Gm | Lab collection (Slack et al. 2006) |
| SMR9811 | ΔuvrD404::FRTcatFRT metE163::Tn10 | Magner et al. (2007) |
| SMR9812 | ΔuvrD404::FRTcatFRT ΔrecQ1906::FRT metE163::Tn10 | Magner et al. (2007) |
| SMR10253 | MG1655 ΔmutS::FRTKanFRT | MG1655 × P1(JW2703) |
| SMR10399 | AB1157 ΔruvABC::cat zea-3::Tn10 | Fonville et al. (2010a) |
| SMR10433 | AB2497 ΔrecA635::FRTKanFRT | Fonville et al. (2010a) |
| SMR10445 | AB2497 ΔmutS::FRTKanFRT | AB2497 × P1(SMR10253) |
| SMR10660 | AB2497 ΔruvABC::cat zea3::Tn10 | Fonville et al. (2010a) |
| SMR10665 | AB2497 ΔrecB::Kan | AB2497 × P1(RTC0013) |
| SMR10669 | AB2497 lexA3 malB::Tn9 | Fonville et al. (2010a) |
| SMR10670 | AB2497 Δ(srlR-recA)306::Tn10 | Fonville et al. (2010a) |
| SMR10671 | AB2497 Δ(srlR-recA)306::Tn10 ΔrecB::Kan | SMR10665 × P1(GY8322) |
| SMR10672 | AB2497 ΔtopB::FRTKanFRT | AB2497 × P1(JW1752) |
| SMR10681 | AB2497 ΔrecQ1906::FRT | Fonville et al. (2010a) |
| SMR10691 | AB2497 ΔrecF1804::FRTKanFRT | Fonville et al. (2010a) |
| SMR10692 | AB2497 lexA3 ΔrecF1804::FRTKanFRT | Fonville et al. (2010a) |
| SMR10913 | AB2497 ΔrecQ1906::FRT ΔrecA635::FRTKanFRT | Fonville et al. (2010a) |
| SMR11118 | AB2497 ΔruvABC::cat zea3::Tn10 ΔrecA635::FRTKanFRT | Fonville et al. (2010a) |
| SMR11193 | AB2497 ΔuvrD404::FRTcatFRT | AB2497 × P1(SMR9811) |
| SMR11194 | AB2497 ΔuvrD404::FRT | SMR11193 × pCP20 |
| SMR11196 | AB2497 ΔrecQ1906::FRT ΔruvABC::cat | SMR10681 × P1(SMR10399) |
| SMR11197 | AB2497 ΔuvrD404::FRTcatFRT ΔrecQ1906::FRT metE163::Tn10 | AB2497 × P1(SMR9812) |
| SMR11199 | AB2497 ΔuvrD404::FRTcatFRT ΔrecA635::FRTKanFRT | SMR11193 × P1(BW26355) |
| SMR11206 | AB2497 ΔuvrD404::FRT ΔmutS::FRTKanFRT | SMR11194 × P1(SMR10253) |
| SMR11207 | AB2497 ΔuvrC403::Gm | AB2497 × P1(SMR8548) |
| SMR11214 | AB2497 ΔrecQ1906::FRT ΔrecB::Kan | SMR10681 × P1(RTC0013) |
| SMR11233 | AB2497 ΔuvrD404::FRTcatFRT ΔrecQ1906::FRT metE163::Tn10 ΔrecA635::FRTKanFRT | SMR11197 × P1(BW26355) |
| SMR11235 | AB2497 ΔrecF1804::FRTKanFRT ΔuvrD404::FRTcatFRT | SMR10691 × P1(SMR9811) |
| SMR11310 | AB2497 recA801 srlC300::Tn10 | SMR10278 × P1(SMR85) |
| SMR11312 | AB2497 ΔrecF1804::FRTKanFRT recA801 srlC300::Tn10 | SMR10691 × P1(SMR85) |
| SMR11314 | AB2497 lexA3 | SMR10669 × P1(DM49) |
| SMR11317 | AB2497 lexA3 ΔuvrD404::FRTcatFRT | SMR11314 × P1(SMR9811) |
| SMR12992 | AB2497 lexA3 ΔrecF1804::FRTKanFRT recA801 srlC300::Tn10 | SMR10692 × P1(SMR85) |
| SMR12994 | AB2497 lexA3 recA801 srlC300::Tn10 | SMR10669 × P1(SMR85) |
| SMR12996 | AB2497 ΔuvrC403::Gm ΔuvrD404::FRTcatFRT | SMR11207 × P1(SMR9811) |
| SMR12998 | AB2497 ΔrecF1804::FRT | SMR10691 × pCP20 |
| SMR12999 | AB2497 ΔrecF1804::FRT ΔuvrD404::FRT | SMR11235 × pCP20 |
| SMR13000 | AB2497 ΔrecF1804::FRT ΔrecA635::FRTKanFRT | SMR12998 × P1(BW26355) |
| SMR13001 | AB2497 ΔrecF1804::FRT ΔuvrD404::FRT ΔrecA635::FRTKanFRT | SMR12999 × P1(BW26355) |
| SMR13003 | AB2497 ΔrecQ1906::FRTΔruvABC::cat ΔrecA635::FRTKanFRT | SMR11196 × P1(BW26355) |
| SMR13005 | AB2497 ΔuvrA402::Gm | AB2497 × P1(SMR8547) |
| SMR13007 | AB2497 ΔuvrA402::Gm ΔuvrD404::FRT | SMR11194 × P1(SMR8547) |
| SMR14220 | AB2497 ΔrecJ::FRTKanFRT | AB2497 × P1(JW2860) |
| SMR14228 | AB2497 ΔrecJ::FRTKanFRT ΔruvABC::cat | SMR14220 × P1(SMR10399) |
| SMR14238 | AB2497 ΔrecJ::FRT | SMR14220 × pCP20 |
| SMR14239 | AB2497 ΔrecJ::FRT ΔuvrD404::FRTcatFRT | SMR14238 × P1(SMR9811) |
| SMR14241 | AB2497 ΔrecJ::FRT ΔuvrD404::FRTcatFRT ΔrecQ1906::FRT metE163::Tn10 | SMR14238 × P1(SMR9812) |
| SMR14242 | AB2497 ΔrecJ::FRT ΔrecF1804::FRTKanFRT | SMR14238 × P1(SMR8097) |
| SMR14244 | AB2497 ΔrecJ::FRT ΔrecA635::FRTKanFRT | SMR14238 × P1(BW26355) |
| SMR14249 | AB2497 ΔrecJ::FRT ΔuvrD404::FRTcatFRT ΔrecF1804::FRTKanFRT | SMR14239 × P1(SMR8097) |
| SMR14251 | AB2497 ΔrecJ::FRT ΔuvrD404::FRTcatFRT ΔrecA635::FRTKanFRT | SMR14239 × P1(BW26355) |
| SMR14253 | AB2497 ΔrecJ::FRT ΔrecQ1801::FRTcatFRT | SMR14238 × P1(SMR6201) |
| SMR14490 | AB2497 ΔrecJ::FRT ΔrecQ1801::FRTcatFRT ΔrecA635::FRTKanFRT | SMR14253 × P1(BW26355) |
CGSC, The E. coli Genetic Stock Center (Yale University).
For microscopy, cultures were started as for TLD assays with stationary cultures diluted 1:20 into 5 ml of fresh thy+ growth medium and incubated at 37° for ∼1 hr until cells had entered early log phase (OD450 ≈ 0.3). DAPI, 2 µg/ml, was added to the cells 10 min prior to washing. One milliliter of cells was washed twice with TLD medium and then resuspended in 0.1 (recB) or 0.5 (parental) ml of TLD medium with 1 µg/ml of DAPI and 1 µg/ml of propidium iodide (PI). Ten microliters of cells was spotted onto a TLD-medium plate (TLD medium solidified with 1.3% agar) and allowed to dry. Agar squares, 1 cm2, containing the spots were cut from the agar and inverted onto a microscope slide. Moist Kim Wipes were placed next to the agar plugs to maintain humidity during the incubation. Microscopy was performed using an Olympus 81× inverted fluorescence microscope with a Hamamatsu HD camera. Cells were maintained at 34° to 36° using a custom-built Precision Weather Station and imaged every 10 min for brightfield and DAPI with PI imaging (TRITC filter) every 30 min. The microscope was set to autofocus every 30 min. Slidebook software was used to program the microscope and to process the images.
Error bars represent 1 SEM of ≥ 3 independent experiments. Statistical analyses were performed using SigmaStat (SYSTAT) and/or SPSS (PASW Statistics) software. For TLD assays significance was determined as P < 0.05 using two-way repeated measure ANOVA to analyze the curve data and Tukey post-hoc analysis.
Results
Two aspects of the TLD protocol were controlled to allow comparisons between strains of different growth rates. First, TLD was reported to be growth-phase dependent: inefficient in stationary phase but efficient in log-phase cells (Kuong and Kuzminov 2010). To allow sufficient time for recB strains, which grow slowly, to exit stationary phase, these were incubated in growth medium for an additional 30 min prior to thymine starvation (Materials and Methods). However, second, we found that cells taken straight from stationary phase and diluted to an OD of 0.3 (the density at which early log-phase cells were subjected to TLD) showed similar or greater sensitivity to TLD than cells in early log phase (supporting information, Figure S1). These data indicate a cell-density effect on TLD and highlight the need to examine cells at constant, low density to see maximal TLD. Thus, all strains were resuspended in TLD medium at ∼5 × 106 cells/ml at 0 min (Materials and Methods).
Removal of DNA-repair intermediates is not how UvrD promotes TLD resistance
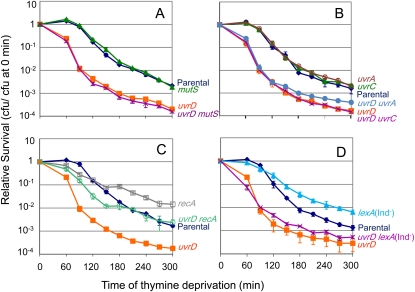
We wished to understand the basis of the hypersensitivity of ΔuvrD mutants to TLD (Siegal 1973). UvrD is a DNA helicase that resolves/removes intermediates in mismatch repair (MMR) and nucleotide excision repair (NER) (Friedberg et al. 2005) and also opposes HR by stripping RecA off ssDNA (Veaute et al. 2005). MMR-defective ΔmutS cells are not affected in TLD (Figure 1A; Kuong and Kuzminov 2010), implying that the absence of MMR ability per se cannot explain the TLD hypersensitivity of ∆uvrD mutants. However, MutS acts early in MMR, by binding DNA mismatches, such that cells that lack MutS do not initiate any MMR reaction. By contrast, UvrD acts after MutS and MutL have bound a DNA mismatch and MutH endonuclease has cleaved DNA near the mismatch, to unwind the DNA, removing the MMR intermediate of protein-bound nicked DNA (Li 2008). Therefore, unlike ∆mutS cells, ∆uvrD single-mutant cells will accumulate DNA mismatches bound by MutS and MutL with single-strand nicks nearby. In Figure 1A, we show that ∆uvrD ∆mutS double mutants, which do not begin MMR, are as hypersensitive to TLD as ∆uvrD single mutants, which begin but fail to complete MMR. We conclude that neither lack of MMR nor accumulation of MMR intermediates is the primary reason for the hyper-TLD of ∆uvrD cells.
Figure 1 .
RecA contributes SOS independently to the hyper-TLD of ΔuvrD cells, but neither NER nor MMR intermediates do. (A) Mismatch-repair intermediates are not the main cause of hyper-TLD of ∆uvrD cells. First, mismatch-repair-defective ΔmutS (SMR10445; solid green triangle) cells are not significantly different from the parental strain (AB2497; solid blue diamond). Second, a ΔuvrD ΔmutS double mutant (SMR11206; purple X) showed the same hypersensitivity to TLD as ΔuvrD (SMR11194; solid orange square) alone, indicating that the accumulation of MMR intermediates created by MutS did not cause most hyper-TLD of ΔuvrD cells. (B) NER intermediates are not the main cause of the hyper-TLD of ∆uvrD cells. NER-defective ΔuvrA (SMR13005; open red circle) and ΔuvrC (SMR11207; solid green triangle) cells are not significantly different from the parental strain (AB2497; solid blue diamond). Neither a ΔuvrD ΔuvrA double mutant (SMR13007; solid blue circle), nor a ΔuvrD ΔuvrC double mutant (SMR12996; purple X) showed less hypersensitivity to TLD than ΔuvrD cells (SMR11194; solid orange square) indicating that UvrABC-generated NER intermediates are not the main cause of the ∆uvrD hyper-TLD. (C) RecA is partially required for the hypersensitivity to TLD of ΔuvrD cells. A ΔuvrD ΔrecA double mutant (SMR11199; open green circle) was not as resistant to TLD as the ΔrecA single mutant (SMR10433; open gray square; P < 0.05), but was significantly more resistant than the ΔuvrD single mutant (SMR11193; solid orange square; P < 0.05). Parental: AB2497; solid blue diamond. (D) The SOS response is not required for the hyper-TLD of ΔuvrD cells. The SOS-blocking lexA(Ind−) allele did not relieve the hypersensitivity of ΔuvrD cells. Strains used from top to bottom: lexA(Ind−) (SMR11314; solid blue triangle), parental (AB2497; solid blue diamond), ΔuvrD lexA(Ind−) (SMR11317; purple X), ΔuvrD (SMR11193; solid orange square). Means ±SEM of three independent experiments.
UvrD also unwinds NER intermediates (Sancar 1996). NER-defective ΔuvrC cells have a TLD sensitivity similar to their parental strain (Figure 1B; Morganroth and Hanawalt 2006) indicating that loss of NER per se does not cause TLD. As with MMR, UvrD works late in NER to unwind the damaged, nicked DNA created by UvrA, UvrB, and UvrC (Sancar 1996). Therefore, UvrABC-initiated NER intermediates will persist in the absence of UvrD and might underlie the hyper TLD sensitivity of ΔuvrD mutants. We find that both ∆uvrA ∆uvrD and ∆uvrC ∆uvrD double mutants, which do not begin NER, are as sensitive as ∆uvrD single mutants, which begin but fail to complete NER (Figure 1B). Therefore, we conclude that, as with MMR, accumulated NER intermediates are not the main cause of hyper-TLD of ΔuvrD cells.
Part of how UvrD resists TLD is by opposing RecA and RecF but not SOS
UvrD helicase removes RecA from ssDNA, opposing HR (Flores et al. 2005; Veaute et al. 2005). If the TLD hypersensitivity of ΔuvrD mutants resulted exclusively from the greater abundance of RecA-DNA filaments (Veaute et al. 2005), then we would expect ∆uvrD ∆recA double mutants to have TLD resistance similar to that of ∆recA cells. In Figure 1C we show that removing RecA from ∆uvrD cells (using a ∆uvrD ∆recA double mutant) alleviates some but not all of the hypersensitivity of ∆uvrD cells to TLD. This appears to differ from recent results that show a complete rescue of the uvrD sensitivity by ∆recA (Kuong and Kuzminov 2010). However, whereas we used a null allele (deletion) of uvrD, the uvrD allele used by Kuong and Kuzminov encodes a truncated 230-amino-acid UvrD protein, which may still contain ATP binding and other activity. Thus, their somewhat different result might reflect altered function or partial activities of the mutant UvrD protein rather than UvrD removal. The data in Figure 1C imply that the increased TLD in strains lacking UvrD results from two separate causes: part from the increased persistence of RecA on DNA when UvrD is absent and part independent of the enhancement of a RecA-dependent TLD pathway.
The RecA-dependent component of the hyper-TLD in ∆uvrD cells could, in principle, be caused by increased HR or an increased SOS response. Cells that lack UvrD show increased spontaneous SOS induction (SaiSree et al. 2000), and SOS induction causes TLD via expression of SulA (Fonville et al. 2010a). However, we find that blocking induction of the SOS response using an uncleavable LexA repressor protein, encoded by lexA3(Ind−), did not alleviate the hypersensitivity of ΔuvrD cells (Figure 1D). The data imply that hyperrecombination not hyper-SOS induction is likely to underlie the RecA-dependent component of the hyper-TLD of ΔuvrD cells.
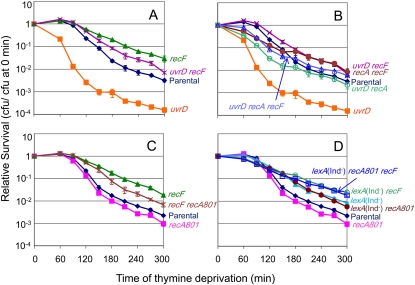
RecF helps RecA load onto ssDNA (Friedberg et al. 2005). If RecF loaded the RecA that promotes the hyper-TLD of ΔuvrD cells, then TLD should be partially blocked in the absence of recF as was seen in the absence of recA. Indeed, Figure 2A shows that ΔrecF partially ameliorates the TLD hypersensitivity of ΔuvrD cells. This again differs from what was seen using a truncated UvrD protein, with which TLD was relieved to a level similar to or greater than that in UvrD+ cells lacking recF (Kuong and Kuzminov 2010). These data suggest that part of the sensitivity of ΔuvrD cells to thymine deprivation might be due to the lack of opposition to RecF-promoted loading of RecA onto ssDNA. To test whether RecF and RecA act via the same (epistatic) or separate (additive) pathways in the absence of uvrD, we compared a ΔuvrD ΔrecA ΔrecF triple mutant with ΔuvrD ΔrecF and ΔuvrD ΔrecA cells. We find that although the ΔuvrD ΔrecA ΔrecF triple mutant is as resistant to TLD as ΔuvrD ΔrecF cells (P = 0.2 at t ≥ 180 min; Figure 2B), as expected, the ΔuvrD ΔrecA ΔrecF triple mutant is significantly more resistant than ΔuvrD ΔrecA cells (P = 0.005 at t ≥ 180 min). These data indicate that RecF promotes TLD via both the RecA-dependent pathway, possibly by loading RecA, and by an additional RecA-independent pathway and mechanism. Because RecF appears not to play the early anti-TLD role that RecA does (Figure 2A and Figure 2B, no early drop in survival of the ΔrecF strains), it seems likely that RecBCD loads RecA in its early anti-TLD role but that RecF loads RecA in its late pro-TLD role. This implies that the early anti-TLD role of RecA is in DSB repair whereas the late pro-TLD role pertains to ssDNA gaps, the RecBCD and RecF substrates, respectively.
Figure 2 .
UvrD action resists two RecF-dependent TLD pathways: one RecA dependent and one RecA independent. (A) RecF is partially required for much but not all of the hypersensitivity of ΔuvrD cells to TLD. A ΔuvrD ΔrecF double mutant (SMR11235; purple X) was more resistant to TLD than ΔuvrD (SMR11193; solid orange square; P < 0.05) alone, but not as resistant as ΔrecF (SMR10691; solid green triangle; P < 0.05). Parental (AB2497; solid blue diamond). (B) RecF and RecA function in independent death pathways in the absence of uvrD. The ΔuvrD ΔrecF (SMR12999; purple X) double mutant is more resistant than the ΔuvrD ΔrecA (SMR11202; open green circle; P < 0.05) mutant indicating that, in ∆uvrD cells, RecF plays a role in TLD in addition to loading RecA. The ΔuvrD ΔrecA ΔrecF triple mutant (SMR13001; open blue triangle) is as resistant to TLD as the ΔuvrD ΔrecF double mutant (P = 0.17) but is more resistant than ΔuvrD ΔrecA cells (P < 0.05). ΔuvrD (SMR11193; solid orange square), ΔrecA ΔrecF (SMR13000; solid brown circle), parental (AB2497; solid blue diamond). Data in A and B are from experiments run in parallel, thus can be compared directly. (C) recA801 partially compensates for RecF in TLD, indicating that part of the RecF role in TLD is loading of RecA. Partial suppression of the ΔrecF phenotype (SMR10691; solid green triangle) by recA801 in the ΔrecF recA801 double mutant (SMR11312; brown X; P < 0.05 compared with ∆recF). Therefore, much of the RecF role in TLD is loading RecA onto ssDNA. recA801 (SMR11310; solid pink square), parental (AB2497; solid blue diamond). The data do not exclude the additional RecA-independent role for RecF in TLD shown in B. (D) The RecA-dependent component of RecF-mediated TLD is via SOS activation. lexA(Ind−) recA801 (SMR12994; solid brown circle) cells are as resistant to TLD as lexA(Ind−) cells (epistatic, same pathway). Thus, the sole role of recA801 in TLD is promoting an SOS response. As expected lexA(Ind−) recA801 ΔrecF (SMR12992; open blue square) cells are more resistant to TLD than the lexA(Ind−) recA801 double mutant (P < 0.05, additive effects), reiterating that RecF functions in a pathway in addition to that of loading RecA and promoting SOS. lexA(Ind−) ΔrecF (SMR10692; open green circle). Parental (AB2497; solid blue diamond), recA801 (SMR11310; solid pink square), lexA(Ind−) (SMR10669; solid blue triangle). Data in C and D are from experiments run in parallel, thus can be compared directly. Means ±SEM of three independent experiments.
RecF promotes TLD by RecA-dependent and RecA-independent pathways
In hyper-TLD of ΔuvrD cells (above), and TLD in UvrD+ cells (Fonville et al. 2010a), RecF and RecA acted in the same pathway, which, in UvrD+ cells, is a pathway of death by induction of the SOS response and SulA (Fonville et al. 2010a). To support the conclusion that part of how RecF promotes TLD in UvrD+ cells is by loading of RecA, and to probe whether SOS induction or HR is the TLD-promoting outcome, we tested whether an allele of recA (recA801) that encodes a RecF-independent RecA protein makes RecF unnecessary. recA801 encodes a RecA protein from a class of mutant RecA’s that compete against single-strand-binding protein (SSB) for binding of ssDNA better than wild-type RecA does (Volkert and Hartke 1987; Madiraju et al. 1992). recA801 compensates for the loss of recF in recombinational DNA repair, but only partially compensates for loss of recF in SOS induction in vivo (Volkert and Hartke 1987). Figure 2C shows that recA801 partially suppresses the TLD resistance of ΔrecF, that is, makes ΔrecF cells more TLD sensitive (ΔrecF recA801 vs. ΔrecF, P < 0.01). The partial suppression of the recF TLD resistance by recA801 might reflect the inability of recA801 to compensate fully for the loss of recF in induction of the SOS response (Volkert and Hartke 1987). We used the SOS-response-blocking lexA(Ind−) mutation to remove induction of the SOS response as a complicating factor and found that recA801 could no longer compensate for the loss of recF in the absence of the SOS response (Figure 2D). This, and the additivity of the effect of blocking SOS with removal of RecF [the lexA(Ind−) ΔrecF double mutant is more resistant than the lexA(Ind−) mutant; Figure 2D; P < 0.05], indicates that although part of the RecF role in promoting TLD is loading RecA onto ssDNA and promoting an SOS response as previously determined (Fonville et al. 2010a), RecF plays an additional RecA/SOS-independent role in promoting TLD, both in the presence and absence of uvrD. One possibility for a RecA-loading/SOS-induction-independent role of RecF in promoting TLD might be RecF-promoted stabilization of stalled replication forks, discussed further below.
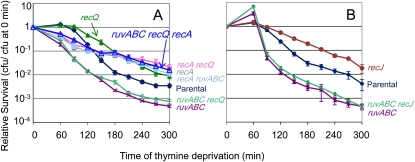
UvrD resists RecQ-dependent and RecQ-independent TLD pathways
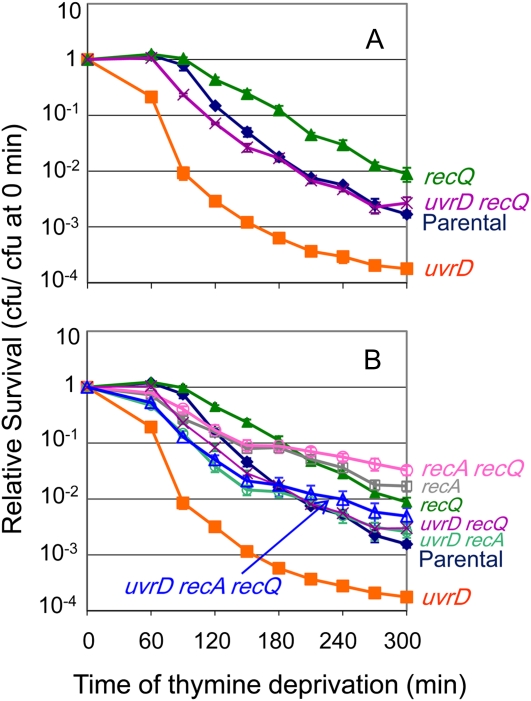
RecQ is required for an SOS-independent pathway of TLD in E. coli (Fonville et al. 2010a). This RecQ-dependent TLD pathway might have promoted part of the hyper-TLD in the absence of uvrD. Indeed, removing recQ partially rescues the hyper-TLD of ΔuvrD cells (Figure 3A). This indicates that one of the TLD pathways that UvrD resists is RecQ promoted. However, in ∆uvrD cells RecQ might promote TLD either through a RecA-dependent pathway of hyperaccumulation of toxic intermolecular HR intermediates as described previously in cells lacking UvrD (Magner et al. 2007; Fonville et al. 2010b) or through a RecA-independent TLD pathway as it does when UvrD is present (Fonville et al. 2010a). We find that the triple ΔuvrD ΔrecA ΔrecQ mutant is not significantly more resistant to TLD than ΔuvrD ΔrecA or the ΔuvrD ΔrecQ cells (Figure 3B; P = 0.99 for both comparisons at t ≥ 180 min). That is, recA and recQ are epistatic. This indicates that the role of RecQ in promoting hyper-TLD in ΔuvrD cells occurs through the same pathway as RecA and supports a death-by-recombination pathway similar to those described previously (Magner et al. 2007; Fonville et al. 2010b). Additionally, we conclude that part of the hyper-TLD observed in ΔuvrD cells occurs via a RecA- and RecQ-independent pathway because triply mutant ΔuvrD ΔrecA ΔrecQ cells were significantly more sensitive to TLD than ΔrecA ΔrecQ double-mutant cells (P ≤ 0.001 at t ≥ 180 min; Figure 3B).
Figure 3 .
(A) UvrD action resists TLD by a RecQ-dependent and a RecQ-independent pathway. RecQ is partially required for the hypersensitivity of ΔuvrD cells to TLD. The ΔuvrD ΔrecQ (SMR11197; purple X) double mutant is more resistant to TLD than ΔuvrD (SMR11193; solid orange square; P < 0.05), but not as resistant as ΔrecQ cells (SMR10681; solid green triangle; P < 0.05). Parental (AB2497; solid blue triangle). (B) The RecQ-dependent pathway that UvrD resists is also RecA dependent, in that RecA and RecQ promote TLD hypersensitivity of ΔuvrD cells via the same (epistatic) pathway. The triple ΔuvrD ΔrecA ΔrecQ mutant was not significantly more resistant to TLD than either the ΔuvrD ΔrecA or ΔuvrD ΔrecQ double mutant, but was not as resistant to TLD as the ΔrecA ΔrecQ double mutant (P < 0.05). Strains from top to bottom: ΔrecA ΔrecQ (SMR10913; open pink circle), ΔrecA (SMR10433; open gray square), ΔrecQ (SMR10681; solid green triangle), ΔuvrD ΔrecA ΔrecQ (SMR11233; open blue triangle), ΔuvrD ΔrecQ (SMR11197; purple X), ΔuvrD ΔrecA (SMR11199; open green circle), parental (AB2497; solid blue triangle); ΔuvrD (SMR11193; solid orange square). Means ±SEM of three independent experiments.
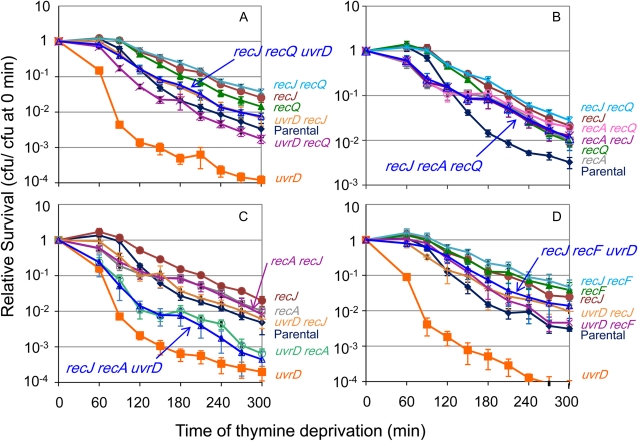
RecJ functions in the RecQ- RecF- RecA-dependent pathway of hyper-TLD in ΔuvrD cells
RecJ is required for the RecQ-dependent pathway of TLD (Fonville et al. 2010a). We show that RecJ is also required for hyper-TLD in ΔuvrD cells (Figure 4A), in which ΔrecJ confers a greater relief of hyper-TLD of ∆uvrD cells (ΔrecJ ΔuvrD; Figure 4A) than does ΔrecQ (ΔrecQ ΔuvrD; Figure 4A, P < 0.01). RecJ might promote the same or a different pathway of hyper-TLD in ΔuvrD cells as RecQ and RecA. First, ΔrecJ ΔrecQ ΔuvrD cells were as sensitive to TLD as ΔrecJ ΔuvrD cells, indicating that RecQ and RecJ do not have additive effects (are epistatic; Figure 4A), but ΔrecJ confers greater resistance than ΔrecQ. We conclude that RecJ and RecQ function in the same pathway of hyper-TLD in ΔuvrD cells, and the greater RecJ effect indicates a possible additional RecQ-independent role of RecJ. Second, ΔrecJ ΔrecA ΔuvrD cells were as sensitive to TLD as ΔrecA ΔuvrD cells, indicating that RecA and RecJ do not have additive effects (Figure 4C). We conclude that RecJ acts in the RecA-dependent pathway of hyper-TLD in ΔuvrD cells. Third, ΔrecJ ΔrecF ΔuvrD cells were as sensitive to TLD as ΔrecJ ΔuvrD cells, not additively so (Figure 4D). This implies that RecJ and RecF act in the same pathway of hyper-TLD in ∆uvrD cells. These data show that unlike the separate RecJ/Q- vs. RecA/F-dependent pathways of TLD in UvrD+ cells (Fonville et al. 2010a), hyper-TLD in ∆uvrD cells is promoted by RecJ, RecQ, RecF, and RecA acting primarily in a single pathway.
Figure 4 .
(A) RecJ is partially required for the hyper-TLD of ΔuvrD cells, and loss of RecJ relieves the hyper-TLD to a greater level than loss of RecQ. The loss of both RecJ and RecQ relieves the hyper-TLD of ΔuvrD cells to the same degree of RecJ alone. Strains from top to bottom: ΔrecJ ΔrecQ (SMR14253; blue asterisk), ΔrecJ (SMR14238; solid brown circle), ΔrecQ (SMR10681; solid green triangle), ΔuvrD ΔrecJ (SMR14239; orange +), ΔrecJ ΔrecQ ΔuvrD (SMR14241; open blue triangle), parental (AB2497; solid blue diamond), ΔuvrD ΔrecQ (SMR11197; purple X), ΔuvrD (SMR11193; solid orange square). (B) ΔrecA ΔrecJ cells were not significantly more resistant to TLD than ΔrecA cells and removing RecQ did not have an additional effect in that ΔrecJ ΔrecA ΔrecQ cells showed no additional TLD resistance above that in ΔrecA ΔrecJ cells. Strains from top to bottom: ΔrecJ ΔrecQ (SMR14253; blue asterisk), ΔrecJ (SMR14238; solid brown circle), ΔrecA ΔrecQ (SMR10913; open pink circle), ΔrecA ΔrecJ (SMR14244; purple X), ΔrecJ ΔrecA ΔrecQ (SMR14490; open blue triangle), ΔrecQ (SMR10681; solid green triangle), ΔrecA (SMR10670; open gray square), parental (AB2497; solid blue diamond). (C) ΔrecJ ΔrecA ΔuvrD cells are as sensitive to TLD as ΔrecA ΔuvrD cells. Strains from top to bottom: ΔrecJ (SMR14238; solid brown circle), ΔrecA (SMR10670; open gray square), ΔrecA ΔrecJ (SMR14244; purple X), ΔuvrD ΔrecJ (SMR14239; orange +), parental (AB2497; solid blue diamond), ΔuvrD ΔrecA (SMR11199; open green circle), ΔrecJ ΔrecA ΔuvrD (SMR14251; open blue triangle), ΔuvrD (SMR11193; solid orange square). (D) ΔrecJ ΔrecF ΔuvrD cells are as resistant to TLD as ΔuvrD ΔrecJ cells, but are more resistant than ΔuvrD ΔrecF cells. Strains from top to bottom: ΔrecJ ΔrecF (SMR14242; blue asterisk), ΔrecF (SMR12998; solid green triangle), ΔrecJ (SMR14238; solid brown circle), ΔrecJ ΔrecF ΔuvrD (SMR14249; open blue triangle), ΔuvrD ΔrecJ (SMR14239; orange +), ΔuvrD ΔrecF (SMR12999; purple X), parental (AB2497; solid blue diamond), ΔuvrD (SMR11193; solid orange square). Means ±SEM of three independent experiments.
RecJ functions in both the RecQ and RecA-dependent TLD pathways in UvrD+ cells
Whereas, RecA, RecF, RecQ, and RecJ act in one linear pathway of hyper-TLD in ΔuvrD cells (Figures 3B and Figure 4, A, C, and D), RecQ and RecJ were shown previously to act in one pathway of TLD in UvrD+ cells while RecA and RecF acted in a second SOS-response-dependent pathway that is independent of RecQ (Fonville et al. 2010a). Whether RecJ might also function in the RecA/F-dependent (RecQ-independent) TLD pathway in UvrD+ cells had not been tested. Figure 4C shows that ΔrecJ cells are slightly more resistant than ΔrecA cells (P = 0.03) and that ΔrecA ΔrecJ cells are similar in resistance to recA suggesting action in the same pathway. The slightly greater resistance of ΔrecJ than ΔrecA and ∆recA ΔrecJ cells might be because all ∆recA cells suffer an early reduction in survival (dip in curves prior to 180 min) that then lowers the point at which the second, resistant phase of ∆recA curves begins (Fonville et al. 2010a; Kuong and Kuzminov 2010), or because RecJ acts partly in a pathway separate from RecA. Either way, these results support the action of RecJ at least partially in the RecA-dependent (RecQ-independent) pathway of TLD in UvrD+ cells. Also supporting this interpretation, ΔrecJ cells are more resistant than ∆recQ (P = 0.01) with the ΔrecJ ∆recQ double mutants resembling ΔrecJ (Figure 4B; Fonville et al. 2010a). Additionally, we find that RecJ and RecF function in a single TLD pathway in that ΔrecJ ΔrecF cells were not significantly more resistant to TLD than ΔrecF cells (Figure 4D; P = 0.86 at t ≥ 180 min), having an epistatic interaction diagnostic of a single pathway, not an additive interaction diagnostic of separate pathways.
The hyper-TLD pathway opposed by RuvABC is RecQ- and RecJ-independent
ΔruvABC cells are hypersensitive to TLD and this hypersensitivity requires RecA (Fonville et al. 2010a). We suggested that death-by-recombination, in which unresolved IRIs block chromosome segregation (Magner et al. 2007), might cause the hyper-TLD of ΔruvABC cells (Fonville et al. 2010a) as it does death of ruv-defective ΔuvrD cells (Magner et al. 2007) and appears to cause part of hyper-TLD in ΔuvrD cells (Figure 1D). RecQ and RecJ promote death-by-recombination in ruv uvrD (Magner et al. 2007) or recG uvrD cells (Fonville et al. 2010b). However, we find that neither ΔrecQ (Figure 5A) nor ΔrecJ (Figure 5B) relieves the hyper-TLD of ΔruvABC cells. Also ΔruvABC ΔrecQ ΔrecA cells are as resistant to TLD as ΔrecA ΔruvABC cells (Figure 5A). These results indicate that although the hyper-TLD in cells lacking ruvABC is RecA dependent and probably occurs via death-by-recombination (Fonville et al. 2010a), neither RecQ nor RecJ plays a role in it, a surprising result discussed below.
Figure 5 .
The RecA-dependent TLD pathway resisted by RuvABC is not RecQ/J-dependent. (A) ΔruvABC ΔrecQ cells (SMR11196; green +) are as hypersensitive to TLD as ΔruvABC cells (SMR10660; purple X); also, triply mutant ΔruvABC ΔrecQ ΔrecA cells (SMR13003; open blue triangle) are as resistant to TLD as ΔrecA ΔruvABC cells (SMR11118; solid blue circle). ΔrecA ΔrecQ (SMR10913; open pink circle), ΔrecQ (SMR10681; solid green triangle), ΔrecA (SMR10433; open gray square), parental (AB2497; solid blue diamond). (B) ΔruvABC ΔrecJ cells (SMR14228; green +) are as hypersensitive to TLD as ΔruvABC cells (SMR10660; purple X). ΔrecJ (SMR14220; solid brown circle), parental (AB2497; solid blue diamond). Means ±SEM of three independent experiments.
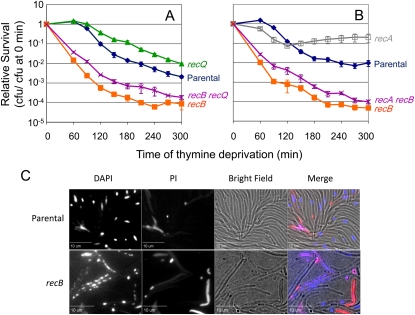
The main role of RecBCD in TLD-resistance is not protection from SOS, recombination, or RecQ-generated DNA ends
Cells lacking RecB, which processes DSEs into substrates for recombination, are hypersensitive to TLD (Nakayama et al. 1982). Previously we suggested that RecQ might promote TLD by creating DSEs generated by overlap of tracts of single-strand degradation by RecQ helicase with RecJ exonuclease at nearby sites (Fonville et al. 2010a). RecB would then degrade these DSEs, possibly removing extra ori-proximal DNA segments from uncompleted chromosome replication and releasing nucleotides that might extend survival. If processing RecQ-generated DSEs were the sole role of RecBCD in TLD resistance, then RecBCD would confer no TLD resistance in cells lacking RecQ. To test whether DSE formation by RecQ is responsible for creating the DNA substrate that then must be processed by RecBCD for resistance to TLD, we examined the sensitivity of a ΔrecB ΔrecQ double mutant and found that it was only slightly, though significantly, more resistant to TLD than ΔrecB (Figure 6A; P < 0.05). This suggests that if RecQ creates some, it does not create most, of the DSEs/DSBs the processing of which by RecBCD allows survival of thymine starvation. That is, RecBCD (i.e., DSE processing) is still required for surviving thymine starvation, even in ΔrecQ cells.
Figure 6 .
RecBCD resists a RecA-, SOS-, and RecQ-independent TLD pathway. (A) Much of the RecB role in TLD survival is independent of RecQ. ΔrecB ΔrecQ (SMR11214; purple X) cells are slightly less sensitive to TLD than ΔrecB cells (SMR10665; solid orange square; P < 0.05). ΔrecQ (SMR10681; solid green triangle), parental (AB2497; solid blue diamond). (B) Neither SOS nor homologous recombination creates the problem that RecB+ action resists in that RecB is required for TLD survival in the absence of RecA. ΔrecA ΔrecB cells (SMR10671; purple X) are slightly less sensitive to TLD than ΔrecB cells (SMR10665; solid orange square; P < 0.05). ΔrecA (SMR10670; open gray square), parental (AB2497; solid blue diamond). Means ±SEM of three independent experiments. (C) Representative pictures of parental (AB2497) and ΔrecB cells (SMR10665) after 12 hr of TLD. Note that although many cells or the Rec+ parent are visible in brightfield, few display compact DAPI-stained nucleoids, whereas nearly all of the recB cells visible in brightfield show strings of small fragmented-looking nucleoids, which were not visible before thymine deprivation. In the merged image, DAPI (DNA stain) is blue and propidium iodide (PI, stain for dead cells) is red.
Additionally, cells that lack RecB fail to repair DSEs. In the absence of RecB, RecJ single-strand-dependent exonuclease appears to be able to prepare DSEs for RecA filament formation and, in cooperation with a DNA helicase, allows induction of an SOS response (Vlasic et al. 2008) and some recombination (Ivancic-Bace et al. 2005). To test whether activation of SOS causes most of the sensitivity of ΔrecB cells to TLD, we asked whether ΔrecB hyper-TLD is RecA dependent. We found that ΔrecA ΔrecB cells showed only slightly but significantly more resistance to thymine deprivation than ΔrecB cells (Figure 6B; P < 0.05). Therefore, neither RecA-promoted recombination nor SOS induction is the main cause of hyper-TLD in ΔrecB mutants. The data imply that RecBC-mediated DNA degradation improves survival during thymine starvation, even in the absence of RecA. The slight increase in TLD resistance of both ΔrecB ΔrecQ cells (Figure 6A) and ΔrecA ΔrecB cells (Figure 6B) over ΔrecB cells is likely to result from the additivity of the RecQ- and RecA-promoted pathways operative in wild-type cells with the more robust alternative hyper-TLD pathway that dominates in ∆recB cells.
The hypothesis that RecBCD double-strand exonuclease activity promotes recovery from TLD was supported further by visualizing nucleoids (bacterial chromosomes) of cells undergoing TLD. Upon thymine depletion, parental cells cease cell division, as seen previously (Fonville et al. 2010a), and the DNA appears diffuse within the cell, i.e., mostly not visible with DAPI, although a subset of cells possess a single compact centrally localized nucleoid (Figure 6C). By contrast, the DNA in ∆recB cells appeared fragmented as many small DAPI foci dispersed throughout the cells (Figure 6C). Such foci were not seen in ∆recB cells before thymine deprivation. RecBCD exonuclease and DSE-repair activities may help maintain the nucleoid, allowing recovery upon plating in the presence of thymine.
Discussion
This study examined the pathways by which UvrD, RuvABC, and RecBCD protect cells from TLD. We sought to identify proteins in the pathways that produce the DNA substrates that kill cells more rapidly in the absence of these DNA-repair proteins.
The anti-TLD role of UvrD
In cells lacking uvrD, we found that the hyper-TLD does not result primarily from either incomplete NER or MMR intermediates (Figure 1, A and B) and partially requires RecA, RecF, RecQ, and RecJ (Figures 1C, 2A, and 3A) acting in a single pathway (Figure 4, A, C, and D), but does not require induction of the SOS response (Figure 1D). These data suggest that the UvrD anti-recombination role (Veaute et al. 2005) could account for protection against the RecA-dependent component of hyper-TLD seen in ΔuvrD cells. This could occur by a death-by-recombination model, such as in Figure 7B, in which unresolved IRIs kill cells by blocking chromosome segregation. Because RecF loads RecA onto ssDNA in single-strand gaps (whereas RecBCD loads RecA onto ssDNA at double-strand ends) (Holthausen et al. 2010), the RecF dependence of this death route implies that ssDNA gaps are the DNA substrate at which most of the UvrD anti-RecA anti-TLD activity is focused. We have drawn these gaps at stalled replication forks in Figure 7, A and B.
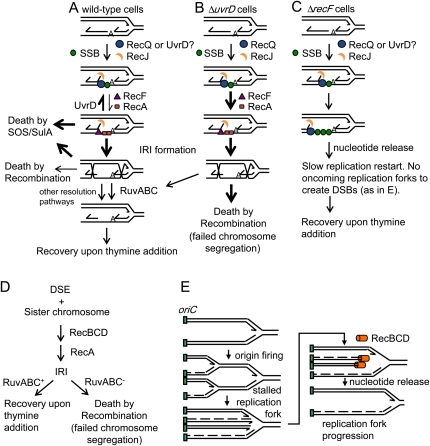
Figure 7 .
Models for UvrD-, RuvABC-, and RecB-promoted resistance to TLD. (A) In the presence of UvrD, its action to oppose RecA filament formation and a possible additional role in opposing the action of RecQ might maintain the majority of stalled replication forks in a manner that would allow them to recover and for replication to proceed when cells are returned to thymine, thus allowing colonies to form. UvrD could do this partly by directly or indirectly inhibiting RecQ action, in that hyper-TLD in ∆uvrD cells is partly RecQ dependent (Figure 3) and by removing RecA from ssDNA thus preventing death-by-recombination. The fraction of replication forks that do become entangled by IRIs require RuvABC for their resolution prior to replication fork restart. (B) In the absence of uvrD, RecQ and RecA are unopposed and the majority of replication forks might then be converted into IRIs, in that few can recover upon re-introduction of thymine and this death is RecA and RecQ dependent (Figures 1 and 3). Death-by-recombination might result if cell division is attempted before all the IRIs are resolved by RuvABC, resulting in tearing of the chromosomes. There is also a RecQ and RecA-independent component to TLD in ΔuvrD cells via an unknown mechanism that is not depicted. (C) Cells lacking recF are resistant to TLD largely due to failure to load RecA onto regions of ssDNA, thus to failure to initiate IRI formation and induce the SOS response (Fonville et al. 2010a). In addition, unwinding and degradation of nascent DNA at stalled replication forks in the absence of RecF might release nucleotides that could be used to prolong survival while leaving ssDNA protected by SSB, potentially explaining the RecA-independent component of RecF-dependent TLD. (D) The RecA dependence but RecQ/J independence of hyper-TLD in RuvABC− (UvrABC+) cells could be explained by a death-by-recombination model in which most of the IRIs formed in UvrABC+ cells and resolved by RuvABC are instigated by DSEs, such that lethal IRIs form without RecQ/J involvement. (E) Model: RecBCD resists TLD by degradation of DNA ends, releasing nucleotides that forestall TLD. This hypothesis can account for RecQ-/RecA-independent production of DSEs during TLD. DSEs might form when an oncoming replication fork collides with a stalled replication fork. The beneficial role of RecB during TLD could be degradation of these DSEs, releasing nucleotides that could be used to advance the stalled fork and, at the same time, RecBCD could prevent nonproductive recombination. Lines represent strands of DNA, arrows represent 3′-DNA ends, dashed lines represent the lagging strands of the oncoming replication forks. Green boxes represent the origin of replication. IRI, interchromosomal recombination intermediate.
Although we found this pathway to account for only part of the hyper-TLD of UvrD− cells, others reported that hyper-TLD in a different uvrD mutant was totally RecA dependent (Kuong and Kuzminov 2010). Whereas we used a complete deletion (null) allele of uvrD, they used an allele that encodes a truncated 230 amino acid UvrD protein, which may still contain ATP binding and other activity. This, or their different growth temperature (28° as opposed to our 37°), or their slightly different minimal medium from ours may account for the different results. Whereas both labs observed the major RecA-dependent hyper-TLD pathway, we also observed an additional RecA-independent hyper-TLD pathway operative in uvrD-null cells.
The ∆ruvABC and other death-by-recombination pathways require RecQ only when UvrD is absent
We provide evidence here that hyperaccumulation of IRIs, which occurs in ΔuvrD cells (Veaute et al. 2005; Magner et al. 2007; Fonville et al. 2010b), contributes to the hyper-TLD of ∆uvrD cells. That is, ∆uvrD cells appear to die a death-by-recombination (illustrated Figure 7B), implied by the RecQ/RecJ/RecF/RecA dependence but SOS independence of their hyper-TLD (Figures 1–4). It is therefore surprising that RecQ was not required for the hyper-TLD of ΔruvABC cells (Figure 5A), which lack IRI-resolution capacity and die a RecA-dependent hyper-TLD most probably also via death-by-recombination (Fonville et al. 2010a). This was surprising because death-by-recombination in IRI-resolution-defective cells was shown previously to be RecQ dependent (Magner et al. 2007); however, in the latter study, those cells lacked UvrD. The lack of a role for RecQ in hyper-TLD by probable death-by-recombination in RuvABC− UvrD+ cells (Figure 5A), despite the requirement for RecQ in death-by-recombination in cells lacking UvrD (Magner et al. 2007; Fonville et al. 2010b), including the ∆uvrD hyper-TLD studied here (Figure 2), can be explained by two nonmutually exclusive hypotheses.
First, the role for RecQ in death-by-recombination might be to promote the net accumulation of a specific IRI-precursor DNA substrate that is normally opposed by UvrD, and so is a minor contributor to IRI formation in UvrD+ cells but a major contributor in UvrD− cells. Figure 7B shows a possible example of this. In it, in the absence of UvrD, RecQ promotes unwinding of the lagging strand of a stalled replication fork, allowing the ssDNA gap created to invade the leading-strand duplex and form an IRI, which must then be resolved. Perhaps UvrD excels at removing RecA from this particular gapped ssDNA-RecA intermediate created by RecQ/J, but is not as robust at removing RecA from RecQ/J-independent IRI precursors, such as DSEs (Figure 7D), which are processed by RecBCD and not RecQ/J. If so, DSEs might be a major source of IRIs resolved by RuvABC in UvrD+ cells and so the major cause of death-by-recombination in UvrD+ cells lacking RuvABC (Figure 7D). By this view, in the absence of UvrD, both RecQ/J and RecA-promoted death-by-recombination will contribute to TLD; however, in UvrD+ RuvABC− cells, RecA-promoted death-by-recombination causes hyper-TLD without help from RecQ/J (as observed; Figure 5) because UvrD opposed the RecQ/J-generated IRI precursors (Figure 7A), leaving other IRI precursors (e.g., DSEs; Figure 7D) to predominate instead.
Note that the general hypothesis here does not demand that the UvrD-resistant IRI precursor is a DSE; that is just one possibility. An alternative possibility is that UvrD strips RecA specifically from ssDNA gaps only at forks, or at fork-lagging strands, and that other ssDNA gaps that form RecQ/J independently predominate in UvrD+ cells and so cause death-by-recombination in UvrD+ RuvABC− cells.
Second, the possibility that UvrD and RecQ share specific DNA substrates was suggested for E. coli (Lestini and Michel 2008) and observed in Deinococcus radiodurans (Bentchikou et al. 2010). Perhaps RecQ is not needed for the RecA/F/J- and SOS-dependent TLD pathway in UvrD+ cells (Fonville et al. 2010a) because UvrD can substitute for RecQ, for example, in unwinding DNA for RecJ single-strand exonuclease activity (Figure 7A). UvrD substitution for RecQ could explain why in UvrD+ cells RecA, RecF (Fonville et al. 2010a), and RecJ (Figure 4B) work together in a TLD pathway that does not require RecQ (Fonville et al. 2010a), and by contrast why RecQ is required for RecA/F/J-dependent death-by-recombination of either ruv or recG cells that lack UvrD (Magner et al. 2007; Fonville et al. 2010b), and RecA/F/J-dependent death-by-recombinational hyper-TLD of UvrD− cells (Figures 3 and 4). This hypothesis might seem not to explain why RecA (Fonville et al. 2010a) but neither RecQ nor RecJ is required for the hyper-TLD death-by-recombination pathway in RuvABC− cells that possess UvrD. If UvrD had simply substituted for RecQ, RecJ might still have been expected to be required per the model in Figure 7A, as it is in RecQ-dependent death by recombination (Magner et al. 2007; Fonville et al. 2010b) and RecQ-dependent TLD in UvrD+ cells (Fonville et al. 2010a). However, perhaps UvrD, but not RecQ, might work with a different 5′ exonuclease that then substitutes for RecJ. Thus, either hypothesis might explain why RecQ is required for apparent death-by-recombination pathways only in the absence of UvrD.
A RecF-promoted TLD pathway independent of RecA
We found that there is a RecF-dependent but RecA-independent pathway of both the hyper-TLD in UvrD− cells (Figure 4D) and TLD in UvrD+ cells (Fonville et al. 2010a). Although one role of RecF could be creation of double-strand DNA ends by nonconservative (nonreciprocal) recombination (Takahashi et al. 1992), this process was RecA-dependent, and so cannot readily account for the RecA-independent role of RecF in TLD described here. Alternatively, RecF was suggested to stabilize stalled replication forks, protecting the nascent lagging strand from degradation and allowing efficient replication restart (Courcelle et al. 1997). In the absence of RecF, restart of stalled replication forks is slowed, and initiation of new rounds of replication from the origin is delayed (Rudolph et al. 2008). The RecF promotion of initiations from the ori after fork stalling might be its RecA-independent TLD-promoting role (Figure 7C), both because the new forks allow more fork-stalling TLD opportunities and because the new forks may create lethal DSEs if they hit stalled forks (Figure 7E). Additionally, the generation of large ssDNA regions in the absence of RecF might oppose TLD by releasing nucleotides including thymidine (Figure 7C).
Roles of RecJ in TLD
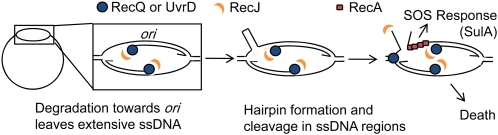
Surprisingly, although we confirmed our previous finding that RecJ and RecQ work in together in a pathway to promote TLD (Fonville et al. 2010a), we discovered that RecJ also participates in the RecA/F/SOS-dependent TLD pathway (Figure 4). These data imply that RecJ functions both with RecA/F (e.g., Figure 7A) as well as with RecQ independently of RecA to promote TLD (e.g., Figure 8). We suggested previously that RecJ and RecQ might promote TLD RecA independently by degrading nascent strands from stalled forks back to the ori, creating large ssDNA regions that could form secondary structures that lead to DSBs and promote death (Fonville et al. 2010a). These might explain the ori-specific DNA loss early in TLD observed both by FISH (Fonville et al. 2010a) and DNA microarrays (Sangurdekar et al. 2010).
Figure 8 .
Model of RecQ/J- or UvrD/RecJ-mediated DNA fragmentation, a possible mechanism for the RecA-independent contribution of RecQ or UvrD to TLD. The RecQ- or UvrD-mediated unwinding of nascent DNA at stalled replication forks toward the ori may lead to RecA-independent DNA destruction. This might be used to restore arrested replication bubbles to the duplex state if the unwinding and RecJ-mediated degradation continues to the opposite stalled fork; however. it also creates extensive regions of ssDNA. Breakage of the DNA, shown here to occur if a hairpin forms and is cleaved by a hairpin endonuclease (but possible with other secondary structures), opens the whole chromosome up to degradation by RecQ or UvrD and RecJ, or RecBCD. Arrows represent 3′-DNA ends.
How RecBCD opposes TLD
Whether hypothetical RecQ/J-promoted DSEs (Figure 8) cause DNA breakage during TLD (Fonville et al. 2010a) is not known. However, our data indicate that if processing such DSEs is how RecBCD avoids TLD, then RecQ is not the sole creator of those DSEs, because hyper-TLD in recB null cells is RecQ independent (Figure 6A). However, given the possible redundancy of UvrD and RecQ helicase activities discussed above, it could be that such DSBs are a major substrate for RecBCD during TLD, but are generated by UvrD when RecQ is absent (Figure 8). We also show that how RecBCD opposes TLD is not via its roles in RecA-promoted recombination or SOS induction because RecB+ protects even RecA− cells from TLD (Figure 6B). One possible model is that the primary role of RecBCD in TLD resistance is degradation of DSEs releasing nucleotides that would otherwise be trapped in DNA and that the released nucleotides forestall eventual TLD. The DSEs could result from new replication forks colliding with stalled forks (Figure 7E), RecQ/UvrD and RecJ action on nascent lagging strands from stalled forks (Figure 8), or replication-fork regression, which occurs at stalled forks creating “chicken-foot” structures with an exposed DSE (Seigneur et al. 1998; not illustrated). All of these models predict the ori-specific DNA loss seen early in TLD in RecBCD+ cells (Fonville et al. 2010a; Sangurdekar et al. 2010).
Alternatively, in cells that lack RecBCD, chicken feet formed at stalled forks persist and so are subject to endonucleolytic cleavage by RuvABC double-strand endonuclease leading to chromosome breakage (Seigneur et al. 1998). Such chicken-foot cleavage might cause hyper-TLD in recB null cells (not illustrated). This would fit with chromosome breakage seen in ∆recBCD cells during TLD (Kuong and Kuzminov 2010) and with the fragmented DAPI-stained nucleoids in ∆recB cells (Figure 6C). Other models are also possible (e.g., Kuong and Kuzminov 2010).
Cancer and chemotherapies
Our findings bear on chemotherapeutic strategies. In addition to mutations in DNA replication proteins, which are associated with TLD resistance in human carcinomas (Yamao et al. 1993), human counterparts of the E. coli DNA repair and damage-response proteins could affect sensitivity importantly. Humans have several RecA homologs including RAD51, the DSB-repair function of which is disrupted in BRCA-defective cells (Moynahan and Jasin 2010), which underlie several cancers (Thacker 2005; Somyajit et al. 2010). Human BRCA2 (Jensen et al. 2010; Liu et al. 2010) and RAD50 (Koroleva et al. 2007) are functional analogs of E. coli RecF. Humans have five RecQ homologs, defects in three of which underlie cancer predisposition syndromes and any of which may be mutated in sporadic cancers (Monnat 2010). Mutations in these genes, others in their pathways, and genes of the DNA-damage response are probable or known in many cancers and might be predicted to confer TLD resistance. Screening cancers for mutations in these genes could help customize more effective chemotherapies, allowing avoidance of TLD-inducing drugs in mutant tumors that are likely to be resistant.
GEN1 and SLX1/SLX4 are human analogs of RuvABC (Ip et al. 2008; Fekairi et al. 2009) and any of several human DNA helicases may function like E. coli UvrD, and so might promote TLD resistance. One might also argue, given the role of the BRCA complex in DSB repair, that the BRCA complex might act more like RecBCD, promoting TLD resistance, than like RecF, promoting TLD, a point that should be tested. Inhibitors designed to target these proteins’ functions might provide powerful adjuncts to TLD-inducing chemotherapies by sensitizing cells to TLD.
Identification of the proteins that cause and protect cells from TLD in bacteria, and their counterparts in humans, is likely to allow customized and improved chemotherapies.
Acknowledgments
We thank Janet Gibson for initiating construction and Ryan Frisch for PCR analysis of recJ mutants. N.C.F. and Z.V. thank Drs. Lei Li and Heidi Kaplan for their support during the manuscript revision and the Department of Microbiology and Molecular Genetics at the University of Texas for the use of the microscope. This work was supported by National Institutes of Health grants R01-GM64022 (P.J.H.) and R01-CA85777 (S.M.R.).
Literature Cited
- Ahmad S. I., Kirk S. H., Eisenstark A., 1998. Thymine metabolism and thymineless death in prokaryotes and eukaryotes. Annu. Rev. Microbiol. 52: 591–625 [DOI] [PubMed] [Google Scholar]
- Anderson J. A., Barbour S. D., 1973. Effect of thymine starvation on deoxyribonucleic acid repair systems of Escherichia coli K-12. J. Bacteriol. 113: 114–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold D. A., Kowalczykowski S. C., 2000. Facilitated loading of RecA protein is essential to recombination by RecBCD enzyme. J. Biol. Chem. 275: 12261–12265 [DOI] [PubMed] [Google Scholar]
- Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., et al. , 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2: 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., 1972. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol. Rev. 36: 525–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barner H. D., Cohen S. S., 1954. The induction of thymine synthesis by T2 infection of a thymine requiring mutant of Escherichia coli. J. Bacteriol. 68: 80–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentchikou E., Servant P., Coste G., Sommer S., 2010. A major role of the RecFOR pathway in DNA double-strand-break repair through ESDSA in Deinococcus radiodurans. PLoS Genet. 6: e1000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner F. R., Plunkett G., 3rd, Bloch C. A., Perna N. T., Burland V., et al. , 1997. The complete genome sequence of Escherichia coli K-12. Science 277: 1453–1474 [DOI] [PubMed] [Google Scholar]
- Cirz R. T., Chin J. K., Andes D. R., de Crecy-Lagard V., Craig W. A., et al. , 2005. Inhibition of mutation and combating the evolution of antibiotic resistance. PLoS Biol. 3: e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J., Sandler S. J., 1994. Homologous genetic recombination: the pieces begin to fall into place. Crit. Rev. Microbiol. 20: 125–142 [DOI] [PubMed] [Google Scholar]
- Courcelle J., Carswell-Crumpton C., Hanawalt P. C., 1997. recF and recR are required for the resumption of replication at DNA replication forks in Escherichia coli. Proc. Natl. Acad. Sci. USA 94: 3714–3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Kusy A. R., 1970. Thymineless death in Escherichia coli: deoxyribonucleic acid replication and the immune state. J. Bacteriol. 102: 106–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko K. A., Wanner B. L., 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97: 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dri A. M., Rouviere-Yaniv J., Moreau P. L., 1991. Inhibition of cell division in hupA hupB mutant bacteria lacking HU protein. J. Bacteriol. 173: 2852–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutreix M., Moreau P. L., Bailone A., Galibert F., Battista J. R., et al. , 1989. New recA mutations that dissociate the various RecA protein activities in Escherichia coli provide evidence for an additional role for RecA protein in UV mutagenesis. J. Bacteriol. 171: 2415–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis D. G., Fisher B., Edmiston S., Mount D. W., 1985. Dual role for Escherichia coli RecA protein in SOS mutagenesis. Proc. Natl. Acad. Sci. USA 82: 3325–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekairi S., Scaglione S., Chahwan C., Taylor E. R., Tissier A., et al. , 2009. Human SLX4 is a Holliday junction resolvase subunit that binds multiple DNA repair/recombination endonucleases. Cell 138: 78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores M. J., Sanchez N., Michel B., 2005. A fork-clearing role for UvrD. Mol. Microbiol. 57: 1664–1675 [DOI] [PubMed] [Google Scholar]
- Fonville N. C., Bates D., Hastings P. J., Hanawalt P. C., Rosenberg S. M., 2010a Role of RecA and the SOS Response in Thymineless Death in Escherichia coli. PLoS Genet. 6: e1000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonville N. C., Blankschien M. D., Magner D. B., Rosenberg S. M., 2010b RecQ-dependent death-by-recombination in cells lacking RecG and UvrD. DNA Repair 9: 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D., 1969. Single-strand breaks in bacterial DNA associated with thymine starvation. J. Mol. Biol. 45: 1–7 [DOI] [PubMed] [Google Scholar]
- Friedberg E., Walker G., Siede W., Wood R., Schultz R., et al. , 2005. DNA Repair and Mutagenesis, Ed. 2 ASM Press, Washington, DC [Google Scholar]
- Holthausen J. T., Wyman C., Kanaar R., 2010. Regulation of DNA strand exchange in homologous recombination. DNA Repair 9: 1264–1272 [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L., 1966. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics 53: 1119–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., 1971. Pleiotropic effect of the recA gene of Escherichia coli: uncoupling of cell division from deoxyribonucleic acid replication. J. Bacteriol. 106: 539–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip S. C., Rass U., Blanco M. G., Flynn H. R., Skehel J. M., et al. , 2008. Identification of Holliday junction resolvases from humans and yeast. Nature 456: 357–361 [DOI] [PubMed] [Google Scholar]
- Ivancic-Bace I., Salaj-Smic E., Brcic-Kostic K., 2005. Effects of recJ, recQ, and recFOR mutations on recombination in nuclease-deficient recB recD double mutants of Escherichia coli. J. Bacteriol. 187: 1350–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. B., Carreira A., Kowalczykowski S. C., 2010. Purified human BRCA2 stimulates RAD51-mediated recombination. Nature 467: 678–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koroleva O., Makharashvili N., Courcelle C. T., Courcelle J., Korolev S., 2007. Structural conservation of RecF and Rad50: implications for DNA recognition and RecF function. EMBO J. 26: 867–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuong K. J., Kuzminov A., 2010. Stalled replication fork repair and misrepair during thymineless death in Escherichia coli. Genes Cells 15: 619–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestini R., Michel B., 2008. UvrD and UvrD252 counteract RecQ, RecJ, and RecFOR in a rep mutant of Escherichia coli. J. Bacteriol. 190: 5995–6001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. M., 2008. Mechanisms and functions of DNA mismatch repair. Cell Res. 18: 85–98 [DOI] [PubMed] [Google Scholar]
- Liu J., Doty T., Gibson B., Heyer W. D., 2010. Human BRCA2 protein promotes RAD51 filament formation on RPA-covered single-stranded DNA. Nat. Struct. Mol. Biol. 17: 1260–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C. R., Yang S., Deibler R. W., Ray S. A., Pennington J. M., et al. , 2005. A role for topoisomerase III in a recombination pathway alternative to RuvABC. Mol. Microbiol. 58: 80–101 [DOI] [PubMed] [Google Scholar]
- Madiraju M. V., Lavery P. E., Kowalczykowski S. C., Clark A. J., 1992. Enzymatic properties of the RecA803 protein, a partial suppressor of recF mutations. Biochemistry 31: 10529–10535 [DOI] [PubMed] [Google Scholar]
- Magner D. B., Blankschien M. D., Lee J. A., Pennington J. M., Lupski J. R., et al. , 2007. RecQ promotes toxic recombination in cells lacking recombination intermediate-removal proteins. Mol. Cell 26: 273–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire J. J., 2003. Anticancer antifolates: current status and future directions. Curr. Pharm. Des. 9: 2593–2613 [DOI] [PubMed] [Google Scholar]
- McPartland A., Green L., Echols H., 1980. Control of recA gene RNA in E. coli: regulatory and signal genes. Cell 20: 731–737 [DOI] [PubMed] [Google Scholar]
- Miller J., 1972. Generalized Transduction: Use of P1 in Strain Construction. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York [Google Scholar]
- Monnat R. J., Jr., 2010. Human RECQ helicases: roles in DNA metabolism, mutagenesis and cancer biology. Semin. Cancer Biol. 20: 329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganroth P. A., Hanawalt P. C., 2006. Role of DNA replication and repair in thymineless death in Escherichia coli. J. Bacteriol. 188: 5286–5288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynahan M. E., Jasin M., 2010. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nat. Rev. Mol. Cell Biol. 11: 196–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H., Hanawalt P., 1975. Sedimentation analysis of deoxyribonucleic acid from thymine-starved Escherichia coli. J. Bacteriol. 121: 537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H., Nakayama K., Nakayama R., Nakayama Y. 1982. Recombination-deficient mutations and thymineless death in Escherichia coli K12: reciprocal effects of recBC and recF and indifference of recA mutations. Can. J. Microbiol. 28: 425–430 [DOI] [PubMed] [Google Scholar]
- Nakayama H., Nakayama K., Nakayama R., Irino N., Nakayama Y., et al. , 1984. Isolation and genetic characterization of a thymineless death-resistant mutant of Escherichia coli K12: identification of a new mutation (recQ1) that blocks the RecF recombination pathway. Mol. Gen. Genet. 195: 474–480 [DOI] [PubMed] [Google Scholar]
- Nakayama K., Shiota S., Nakayama H., 1988. Thymineless death in Escherichia coli mutants deficient in the RecF recombination pathway. Can. J. Microbiol. 34: 905–907 [DOI] [PubMed] [Google Scholar]
- Nakayama K., Kusano K., Irino N., Nakayama H., 1994. Thymine starvation-induced structural changes in Escherichia coli DNA: detection by pulsed field gel electrophoresis and evidence for involvement of homologous recombination. J. Mol. Biol. 243: 611–620 [DOI] [PubMed] [Google Scholar]
- Pennington J. M., Rosenberg S. M., 2007. Spontaneous DNA breakage in single living Escherichia coli cells. Nat. Genet. 39: 797–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph C. J., Upton A. L., Lloyd R. G., 2008. Maintaining replication fork integrity in UV-irradiated Escherichia coli cells. DNA Repair 7: 1589–1602 [DOI] [PubMed] [Google Scholar]
- SaiSree L., Reddy M., Gowrishankar J., 2000. lon incompatibility associated with mutations causing SOS induction: null uvrD alleles induce an SOS response in Escherichia coli. J. Bacteriol. 182: 3151–3157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., 1996. DNA excision repair. Annu. Rev. Biochem. 65: 43–81 [DOI] [PubMed] [Google Scholar]
- Sangurdekar D. P., Hamann B. L., Smirnov D., Srienc F., Hanawalt P. C., et al. , 2010. Thymineless death is associated with loss of essential genetic information from the replication origin. Mol. Microbiol. [DOI] [PubMed] [Google Scholar]
- Seigneur M., Bidnenko V., Ehrlich S. D., Michel B., 1998. RuvAB acts at arrested replication forks. Cell 95: 419–430 [DOI] [PubMed] [Google Scholar]
- Siegal E., 1973. Ultraviolet-sensitive mutator strain of Escherichia coli K-12. J. Bacteriol. 113: 145–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack A., Thornton P. C., Magner D. B., Rosenberg S. M., Hastings P. J., 2006. On the mechanism of gene amplification induced under stress in Escherichia coli. PLoS Genet. 2: e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somyajit K., Subramanya S., Nagaraju G., 2010. RAD51C: a novel cancer susceptibility gene is linked to Fanconi anemia and breast cancer. Carcinogenesis 31: 2031–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N. K., Yamamoto K., Kitamura Y., Luo S. Q., Yoshikura H., et al. , 1992. Nonconservative recombination in Escherichia coli. Proc. Natl. Acad. Sci. USA 89: 5912–5916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura Y., Jackman A. L., 1997. Folate-based thymidylate synthase inhibitors in cancer chemotherapy. Anticancer Drugs 8: 3–16 [DOI] [PubMed] [Google Scholar]
- Thacker J., 2005. The RAD51 gene family, genetic instability and cancer. Cancer Lett. 219: 125–135 [DOI] [PubMed] [Google Scholar]
- Veaute X., Delmas S., Selva M., Jeusset J., Le Cam E., et al. , 2005. UvrD helicase, unlike Rep helicase, dismantles RecA nucleoprotein filaments in Escherichia coli. EMBO J. 24: 180–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasic I., Ivancic-Bace I., Imesek M., Mihaljevic B., Brcic-Kostic K., 2008. RecJ nuclease is required for SOS induction after introduction of a double-strand break in a RecA loading deficient recB mutant of Escherichia coli. Biochimie 90: 1347–1355 [DOI] [PubMed] [Google Scholar]
- Volkert M. R., Hartke M. A., 1987. Effects of the Escherichia coli recF suppressor mutation, recA801, on recF-dependent DNA-repair associated phenomena. Mutat. Res. 184: 181–186 [DOI] [PubMed] [Google Scholar]
- Yamao F., Nagai Y., Kaneda S., Yoshida S., Seno T., 1993. Conditional resistance to thymineless death predominantly selects DNA synthesis-deficient mutants of mammalian cells. Mutat. Res. 289: 83–89 [DOI] [PubMed] [Google Scholar]